 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
| ILR-H82F4 | Human | Biotinylated Human IL-4 R alpha / CD124 Protein, Fc,Avitag™ (MALS verified) |  |

|
|
| ILR-H5253 | Human | Human IL-4 R alpha / CD124 Protein, Fc Tag (MALS verified) |  |

|
|
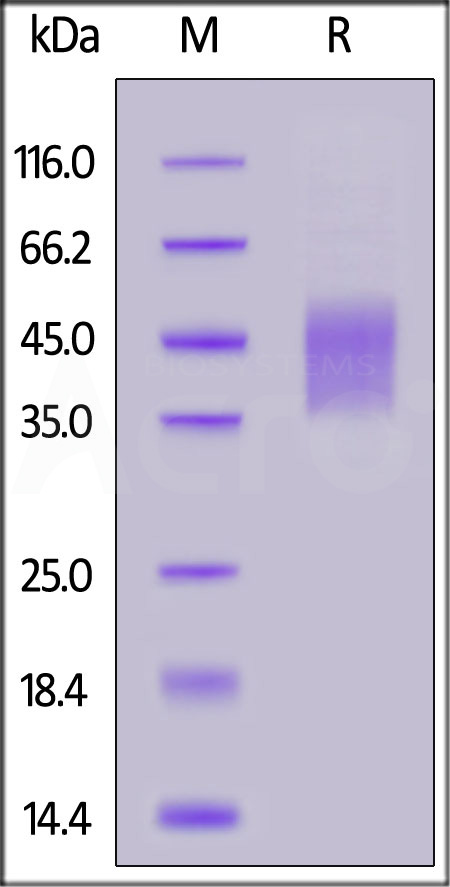
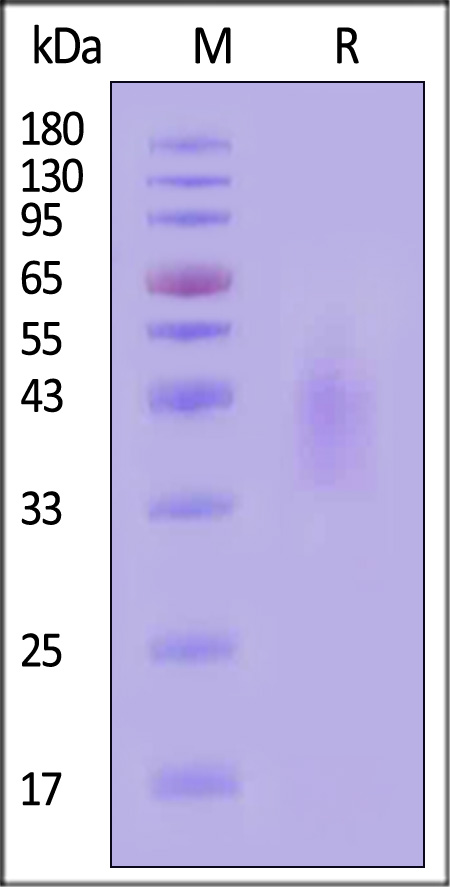
| ILR-H82E9 | Human | Biotinylated Human IL-4 R alpha / CD124 Protein, Avitag™,His Tag (MALS verified) |  |
|
|
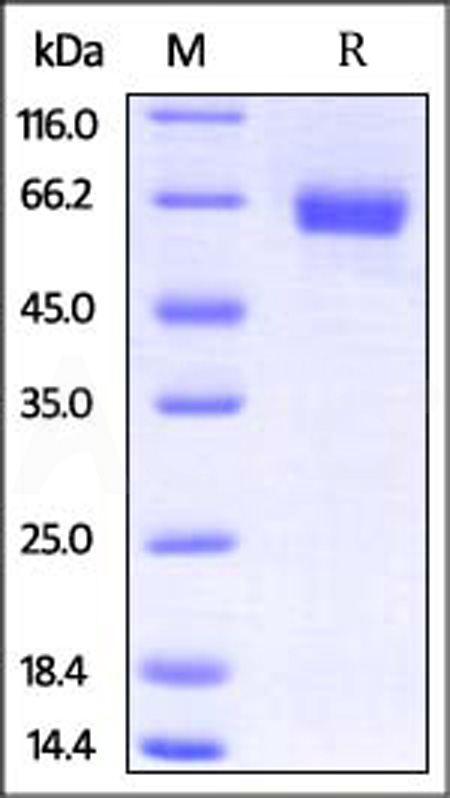
| ILR-M5252 | Mouse | Mouse IL-4 R alpha / CD124 Protein, Fc Tag (MALS verified) |  |
|
|
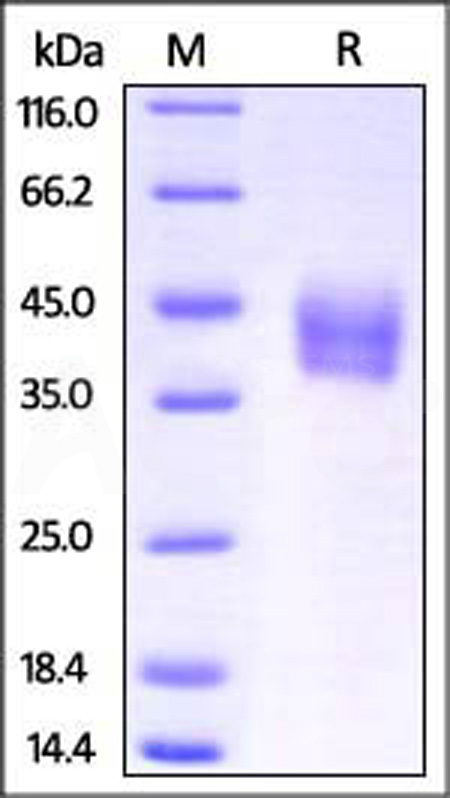
| ILR-M52H1 | Mouse | Mouse IL-4 R alpha / CD124 Protein, His Tag |  |
|
|
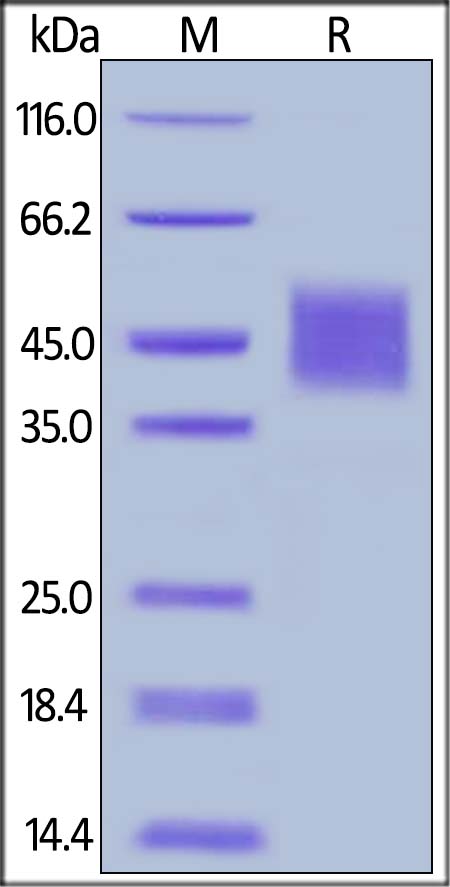
| ILR-C52H8 | Cynomolgus / Rhesus macaque | Cynomolgus IL-4 R alpha / CD124 Protein, His Tag (MALS verified) |  |
|
|
| ILR-C5258 | Cynomolgus / Rhesus macaque | Cynomolgus / Rhesus macaque IL-4 R alpha / CD124 Protein, Fc Tag |  |
|
|
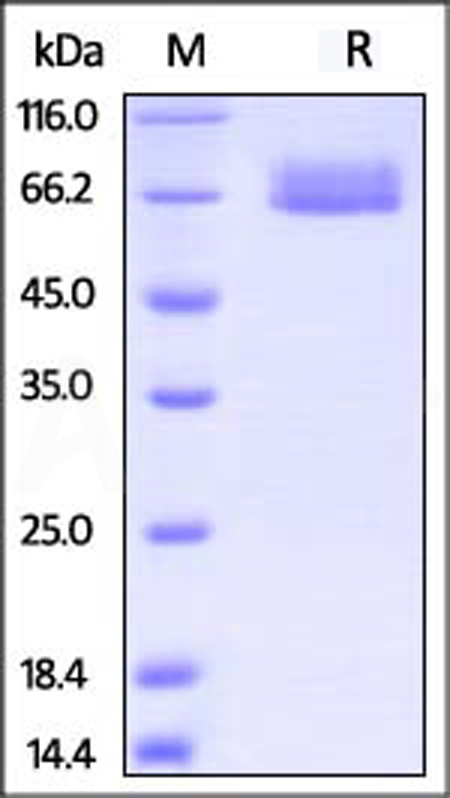
| ILR-H5221 | Human | Human IL-4 R alpha / CD124 Protein, His Tag (MALS verified) |  |
|
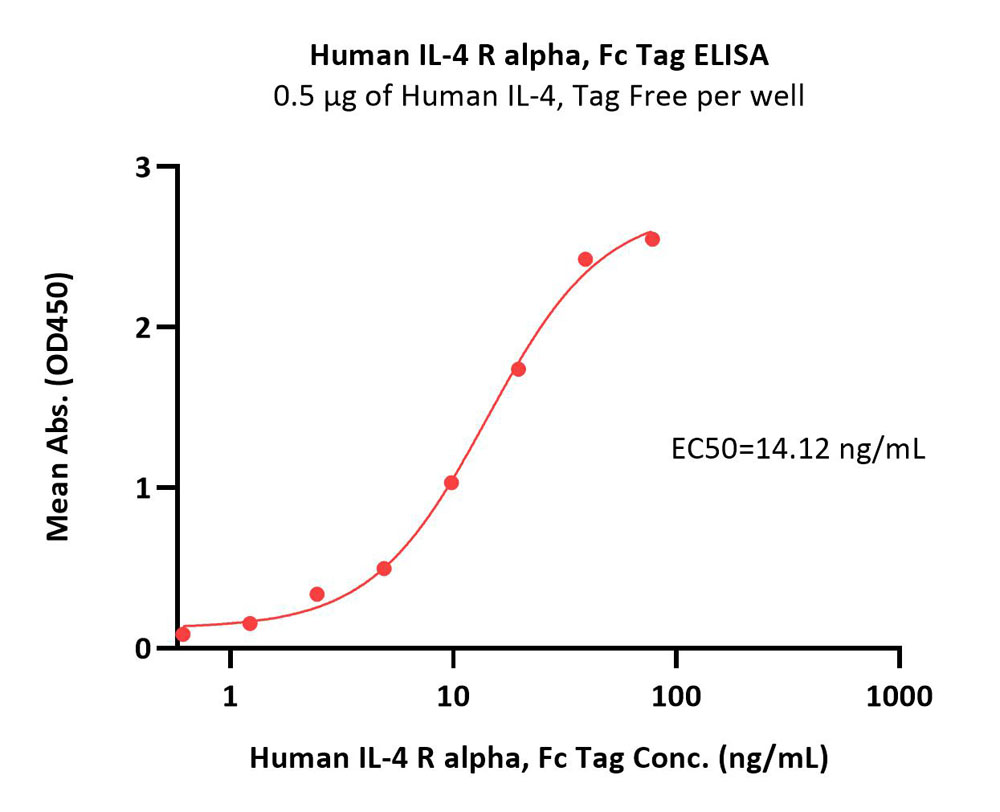
Immobilized Human IL-4, premium grade (Cat. No. IL4-H4218) at 5 μg/mL (100 μL/well)can bind Human IL-4 R alpha, Fc Tag (Cat. No. ILR-H5253) with a linear range of 1-20 ng/mL (QC tested).
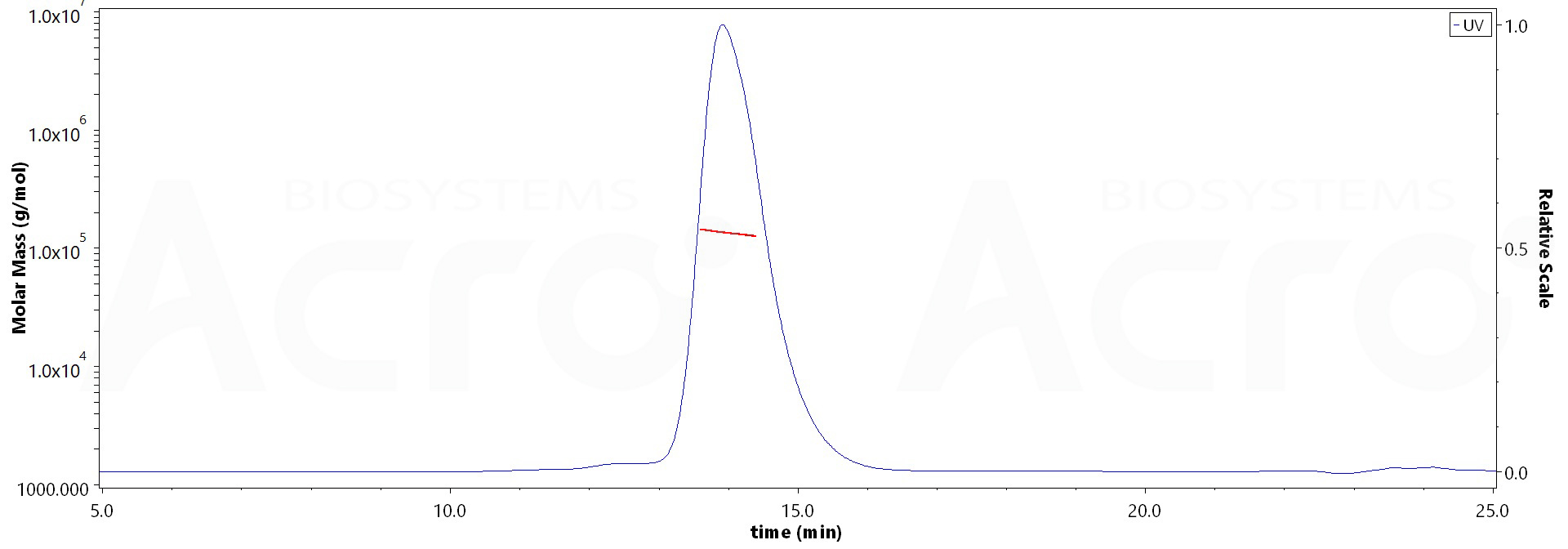
The purity of Biotinylated Human IL-4 R alpha, Fc,Avitag (Cat. No. ILR-H82F4) is more than 90% and the molecular weight of this protein is around 120-147 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Dupilumab | SAR-231893; REGN-668 | Approved | Sanofi | Dupixent, 达必妥 | United States | Dermatitis, Atopic | Regeneron Pharmaceuticals Inc | 2017-03-28 | Asthma, Aspirin-Induced; Sinusitis; Asthma; Duodenitis; Pemphigoid, Bullous; Neurodermatitis; Colitis, Ulcerative; Urticaria; Prurigo; Pulmonary Disease, Chronic Obstructive; Paranasal Sinus Diseases; Sleep Apnea Syndromes; Peanut Hypersensitivity; Keloid; Scleroderma, Localized; Keratoconjunctivitis; Hypersensitivity, Immediate; Dermatitis, Atopic; Angioedema; Eczema; Hypersensitivity; Milk Hypersensitivity; Aspergillosis, Allergic Bronchopulmonary; Pruritus; Gastrointestinal Diseases; Respiratory Sounds; Alopecia Areata; Meningitis; Nasal Polyps; Chronic Urticaria; Respiratory Tract Diseases; Respiration Disorders; Eosinophilic gastroenteritis (EG); Coronavirus Disease 2019 (COVID-19); Dermatitis; Skin Diseases; Skin Diseases, Eczematous; Genetic Diseases, Inborn; Eosinophilic Esophagitis; Prostatic Neoplasms; Conjunctivitis, Allergic; Rhinitis, Allergic | Details |
| Dupilumab | SAR-231893; REGN-668 | Approved | Sanofi | Dupixent, 达必妥 | United States | Dermatitis, Atopic | Regeneron Pharmaceuticals Inc | 2017-03-28 | Asthma, Aspirin-Induced; Sinusitis; Asthma; Duodenitis; Pemphigoid, Bullous; Neurodermatitis; Colitis, Ulcerative; Urticaria; Prurigo; Pulmonary Disease, Chronic Obstructive; Paranasal Sinus Diseases; Sleep Apnea Syndromes; Peanut Hypersensitivity; Keloid; Scleroderma, Localized; Keratoconjunctivitis; Hypersensitivity, Immediate; Dermatitis, Atopic; Angioedema; Eczema; Hypersensitivity; Milk Hypersensitivity; Aspergillosis, Allergic Bronchopulmonary; Pruritus; Gastrointestinal Diseases; Respiratory Sounds; Alopecia Areata; Meningitis; Nasal Polyps; Chronic Urticaria; Respiratory Tract Diseases; Respiration Disorders; Eosinophilic gastroenteritis (EG); Coronavirus Disease 2019 (COVID-19); Dermatitis; Skin Diseases; Skin Diseases, Eczematous; Genetic Diseases, Inborn; Eosinophilic Esophagitis; Prostatic Neoplasms; Conjunctivitis, Allergic; Rhinitis, Allergic | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| siLR4A | siLR4A | Phase 3 Clinical | National Skin Centre, Singapore | Keloid; Wounds and Injuries | Details |
| GR-1802 | GR-1802; GR1802 | Phase 3 Clinical | Genrix (Shanghai) Biopharmaceutical Co Ltd | Rhinitis, Allergic, Seasonal; Nasal Polyps; Nose Diseases; Chronic Urticaria; Rhinitis, Allergic; Sinusitis; Asthma; Dermatitis, Atopic | Details |
| Manfidokimab | AK-120 (Akeso ) | Phase 3 Clinical | Zhongshan Akeso Biopharma Co Ltd | Asthma; Dermatitis, Atopic | Details |
| Comekibart | MG-010; MG-K-10; BC-005; MG-K10 | Phase 3 Clinical | Dragonboat Biopharmaceutical | Nasal Polyps; Asthma; Sinusitis; Dermatitis, Atopic | Details |
| Recombinant anti-IL-4Rα humanized monoclonal antibody (Sansheng Guojian) | 611; 611 Q2W; 611 Q4W; 611-3SBio; SSGJ-611 | Phase 3 Clinical | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | Nasal Polyps; Polyps; Sinusitis; Pulmonary Disease, Chronic Obstructive; Dermatitis, Atopic | Details |
| Rademikibart | CBP-201 | Phase 2 Clinical | Suzhou Connect Biopharmaceuticals Ltd | Nasal Polyps; Nose Diseases; Iron deficiency; Sinusitis; Status Asthmaticus; Asthma; Dermatitis, Atopic | Details |
| QX-005N | QX-005N | Phase 2 Clinical | Qyuns Therapeutics Co Ltd | Chronic Urticaria; Nasal Polyps; Prurigo; Asthma; Sinusitis; Pulmonary Disease, Chronic Obstructive; Dermatitis, Atopic | Details |
| BA-2101 | BA-2101; BA2101; PR-103; PR103 | Phase 2 Clinical | Pruritus; Chronic Urticaria; Nasal Polyps; Sinusitis; Asthma; Prurigo; Urticaria; Pulmonary Disease, Chronic Obstructive; Dermatitis, Atopic | Details | |
| Sorafenib Tosylate/Comekibart | MG-D-1609 | Phase 2 Clinical | Metagone Biotech Inc | Solid tumours | Details |
| TQH2722 | TQH-2722; TQH2722 | Phase 2 Clinical | Nanjing Shunxin Pharmaceuticals Co Ltd Of Chiatai Tianqing Pharmaceutical Group | Nasal Polyps; Sinusitis; Dermatitis, Atopic | Details |
| LQ-036 | LQ-036 | Phase 2 Clinical | Shanghai Novamab Biopharmaceuticals Co Ltd | Asthma; Pulmonary Disease, Chronic Obstructive | Details |
| SHR-1819 | SHR-1819 | Phase 2 Clinical | Atridia Pty Ltd | Nasal Polyps; Sinusitis; Asthma; Dermatitis, Atopic | Details |
| Bizaxofusp | PRX-321; MDNA-55; IL4-PE; NBI-3001 | Phase 2 Clinical | Neurocrine Biosciences Inc, National Institute of Health, Islamabad | Kidney Neoplasms; HIV Infections; Glioblastoma; Small Cell Lung Carcinoma; Central Nervous System Neoplasms; Brain Neoplasms; Breast Neoplasms; Lung Neoplasms; Glioma; Sarcoma, Kaposi | Details |
| Elarekibep | PRS-060/AZD1402; PRS-060; AZD-1402 | Phase 1 Clinical | University Of Melbourne, Pieris Pharmaceuticals | Asthma | Details |
| NM26-2198 | NM-26-2198 | Phase 1 Clinical | Kaken Pharmaceutical Co Ltd, Numab Therapeutics Ag | Inflammation; Dermatitis, Atopic | Details |
| IBI-3002 | IBI3002; IBI-3002 | Phase 1 Clinical | Innovent Biologics(Suzhou) Co Ltd | Asthma | Details |
| APG-808 | APG808; PR-001; PR001; APG-808 | Phase 1 Clinical | Paragon Therapeutics Inc, Apogee Therapeutics Inc | Pulmonary Disease, Chronic Obstructive | Details |
| RC-1416 | Phase 1 Clinical | Nanjing Rongjiekang Biotechnology Co Ltd | Asthma | Details | |
| HY-1770 | HY1770; HT-17; HT17; HY-1770 | Phase 1 Clinical | Suzhou Pharmavan Co Ltd | Dermatitis, Atopic; Plaque psoriasis | Details |
| siLR4A | siLR4A | Phase 3 Clinical | National Skin Centre, Singapore | Keloid; Wounds and Injuries | Details |
| GR-1802 | GR-1802; GR1802 | Phase 3 Clinical | Genrix (Shanghai) Biopharmaceutical Co Ltd | Rhinitis, Allergic, Seasonal; Nasal Polyps; Nose Diseases; Chronic Urticaria; Rhinitis, Allergic; Sinusitis; Asthma; Dermatitis, Atopic | Details |
| Manfidokimab | AK-120 (Akeso ) | Phase 3 Clinical | Zhongshan Akeso Biopharma Co Ltd | Asthma; Dermatitis, Atopic | Details |
| Comekibart | MG-010; MG-K-10; BC-005; MG-K10 | Phase 3 Clinical | Dragonboat Biopharmaceutical | Nasal Polyps; Asthma; Sinusitis; Dermatitis, Atopic | Details |
| Recombinant anti-IL-4Rα humanized monoclonal antibody (Sansheng Guojian) | 611; 611 Q2W; 611 Q4W; 611-3SBio; SSGJ-611 | Phase 3 Clinical | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | Nasal Polyps; Polyps; Sinusitis; Pulmonary Disease, Chronic Obstructive; Dermatitis, Atopic | Details |
| Rademikibart | CBP-201 | Phase 2 Clinical | Suzhou Connect Biopharmaceuticals Ltd | Nasal Polyps; Nose Diseases; Iron deficiency; Sinusitis; Status Asthmaticus; Asthma; Dermatitis, Atopic | Details |
| QX-005N | QX-005N | Phase 2 Clinical | Qyuns Therapeutics Co Ltd | Chronic Urticaria; Nasal Polyps; Prurigo; Asthma; Sinusitis; Pulmonary Disease, Chronic Obstructive; Dermatitis, Atopic | Details |
| BA-2101 | BA-2101; BA2101; PR-103; PR103 | Phase 2 Clinical | Pruritus; Chronic Urticaria; Nasal Polyps; Sinusitis; Asthma; Prurigo; Urticaria; Pulmonary Disease, Chronic Obstructive; Dermatitis, Atopic | Details | |
| Sorafenib Tosylate/Comekibart | MG-D-1609 | Phase 2 Clinical | Metagone Biotech Inc | Solid tumours | Details |
| TQH2722 | TQH-2722; TQH2722 | Phase 2 Clinical | Nanjing Shunxin Pharmaceuticals Co Ltd Of Chiatai Tianqing Pharmaceutical Group | Nasal Polyps; Sinusitis; Dermatitis, Atopic | Details |
| LQ-036 | LQ-036 | Phase 2 Clinical | Shanghai Novamab Biopharmaceuticals Co Ltd | Asthma; Pulmonary Disease, Chronic Obstructive | Details |
| SHR-1819 | SHR-1819 | Phase 2 Clinical | Atridia Pty Ltd | Nasal Polyps; Sinusitis; Asthma; Dermatitis, Atopic | Details |
| Bizaxofusp | PRX-321; MDNA-55; IL4-PE; NBI-3001 | Phase 2 Clinical | Neurocrine Biosciences Inc, National Institute of Health, Islamabad | Kidney Neoplasms; HIV Infections; Glioblastoma; Small Cell Lung Carcinoma; Central Nervous System Neoplasms; Brain Neoplasms; Breast Neoplasms; Lung Neoplasms; Glioma; Sarcoma, Kaposi | Details |
| Elarekibep | PRS-060/AZD1402; PRS-060; AZD-1402 | Phase 1 Clinical | University Of Melbourne, Pieris Pharmaceuticals | Asthma | Details |
| NM26-2198 | NM-26-2198 | Phase 1 Clinical | Kaken Pharmaceutical Co Ltd, Numab Therapeutics Ag | Inflammation; Dermatitis, Atopic | Details |
| IBI-3002 | IBI3002; IBI-3002 | Phase 1 Clinical | Innovent Biologics(Suzhou) Co Ltd | Asthma | Details |
| APG-808 | APG808; PR-001; PR001; APG-808 | Phase 1 Clinical | Paragon Therapeutics Inc, Apogee Therapeutics Inc | Pulmonary Disease, Chronic Obstructive | Details |
| RC-1416 | Phase 1 Clinical | Nanjing Rongjiekang Biotechnology Co Ltd | Asthma | Details | |
| HY-1770 | HY1770; HT-17; HT17; HY-1770 | Phase 1 Clinical | Suzhou Pharmavan Co Ltd | Dermatitis, Atopic; Plaque psoriasis | Details |
This web search service is supported by Google Inc.
