 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
| PTR-H5222 | Human | Human PTH1R / PTHR1 Protein, His Tag |  |
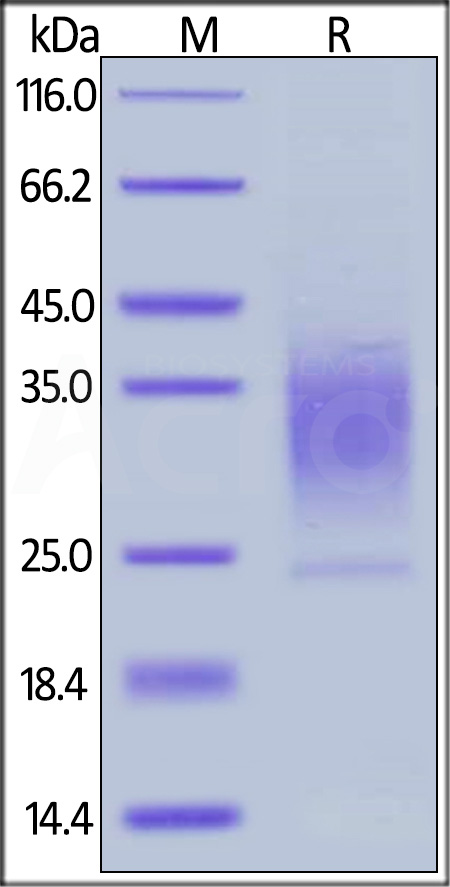
|
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Palopegteriparatide | ACP-014; ACP014; ACP 014 | Approved | Ascendis Pharma | TransCon, YORVIPATH | EU | Hypoparathyroidism | Ascendis Pharma, Ascendis Pharma Bone Diseases As | 2023-11-17 | Hypoparathyroidism | Details |
| Teriparatide biosimilar (Biosidus) | rhPTH (1-34) (Biosidus) | Approved | Biosidus | Osteofortil | Argentina | Osteoporosis | Biosidus | 2012-08-01 | Osteoporosis | Details |
| Abaloparatide | ITM-058; BA-058-SC; BA-058-TD; BIM-44058; BA-058 | Approved | Ipsen | ELADYNOS, Tymlos | United States | Osteoporosis, Postmenopausal | Radius Health Inc | 2017-04-28 | Osteoporotic Fractures; Osteoporosis; Osteoporosis, Postmenopausal; Intervertebral Disc Degeneration; Fractures, Bone | Details |
| Teriparatide biosimilar (Amega Biotech) | rhPTH (1-34) (Amega Biotech) | Approved | Amega Biotech | Osteoporosis | Details | |||||
| Teriparatide biosimilar (Pfenex) | rhPTH (1-34) (Pfenex); PF-708 | Approved | Pfenex Biopharmaceuticals Inc | Bonsity, Qutavina | United States | Osteoporosis, Postmenopausal; Glucocorticoid-induced osteoporosis; Osteoporosis | Alvogen | 2019-10-04 | Osteoporosis; Osteoporosis, Postmenopausal; Glucocorticoid-induced osteoporosis | Details |
| Teriparatide | MN-10T; LY 333334; hPTH-1-34; EB-613 | Approved | Eli Lilly And Company | Forteo, Forsteo, 复泰奥 | United States | Osteoporosis | Eli Lilly And Company | 2002-11-26 | Ankle Fractures; Chronic Kidney Disease-Mineral and Bone Disorder; Anorexia Nervosa; Hypoparathyroidism; Periodontitis; Fractures, Bone; Osteoarthritis, Knee; Back Pain; Spinal Fractures; Humeral Fractures; Femoral Neck Fractures; Cartilage Diseases; Hypocalcemia; Colles' Fracture; Osteoporosis, Postmenopausal; Menopausal symptoms; Spinal Cord Injuries; Bone Diseases, Metabolic; Osteoporosis; Alveolar Bone Loss; Hyperparathyroidism, Secondary; Drug-Related Side Effects and Adverse Reactions | Details |
| Parathyroid hormone (Shire) | NPSP-795; rhPTH (1-84); rhPTH-1-84; PTH (1-84); SB-423562; PTH 1-84; NPSP-558; SHP-635; SHP-634; ALX-111; 423562; EBP-05; EB-612 | Approved | Shire | Natpara, Preotact, Natpar | United States | Hypoparathyroidism | Nps Pharmaceuticals, Inc | 2006-04-24 | Osteoporosis; Bone Diseases, Endocrine; Osteoporosis, Postmenopausal; Hypocalcemia; Psoriasis; Hypoparathyroidism; Hyperparathyroidism | Details |
| Teriparatide biosimilar (Intas Pharmaceuticals) | rhPTH (1-34) (Intas Pharmaceuticals); INTG8; INTG-8 | Approved | Intas Biopharmaceuticals | Terifrac | India | Osteoporosis | Intas Biopharmaceuticals | 2010-12-01 | Osteoporosis | Details |
| Teriparatide biosimilar (Gedeon Richter) | rhPTH (1-34) (Gedeon Richter) | Approved | Gedeon Richter Plc | Terrosa | EU | Osteoporosis | Gedeon Richter Plc | 2017-01-04 | Osteoporosis; Fractures, Stress | Details |
| Teriparatide biosimilar (Salubris) | SAL056; G56-W1; SAL-001 | Approved | Shenzhen Salubris Pharmaceuticals Co Ltd | 欣复泰 | Mainland China | Osteoporosis, Postmenopausal | Suzhou Genemen Biotech Co Ltd | 2019-09-29 | Osteoporosis, Postmenopausal | Details |
| Teriparatide biosimilar (USV) | Approved | Usv Ltd | Osteoporosis | Details | ||||||
| Teriparatide biosimilar (Strides Pharma) | SBL-001 | Approved | Strides Pharma | Kauliv | EU | Osteoporosis; Osteoporosis, Postmenopausal | Strides Pharma (Cyprus) Ltd | 2023-01-12 | Osteoporosis; Osteoporosis, Postmenopausal | Details |
| Teriparatide biosimilar (STADA Arzneimittel AG) | rhPTH (1-34) (STADA Arzneimittel AG) | Approved | Stada Arzneimittel Ag | Movymia | EU | Osteoporosis | Stada Arzneimittel Ag | 2017-01-11 | Osteoporosis | Details |
| Recombinant parathyroid hormone biosimilar(Chongqing Fujin Biological Medicine) | Approved | Chongqing Fujin Biological Medicine Co Ltd | Details | |||||||
| Recombinant Human Parathyroid Hormone (1-34), Shanghai Fosunpharma | Approved | Shanghai Fosun Pharmaceutical (Group) Co Ltd | Details | |||||||
| Teriparatide acetate | hPTH (1-34) acet. H2O; AK-159; MN-10-T | Approved | Union Square Ventures Llc | Parathar, Teribone | Japan | Osteoporosis; Hypocalcemia | null | 1987-03-31 | Osteoporosis; Hypocalcemia | Details |
| Teriparatide biosimilar (Theramex) | Approved | Theramex Ireland Ltd | Livogiva | EU | Osteoporosis | Theramex Ireland Ltd | 2020-08-27 | Osteoporosis | Details | |
| Teriparatide biosimilar (Shanghai United Cell ) | Approved | Shanghai United Cell Biotechnology Co Ltd | Mainland China | Osteoporosis | Shanghai United Cell Biotechnology Co Ltd | 2017-03-22 | Osteoporosis | Details | ||
| Teriparatide biosimilar (Beijing Bokangjian Gene) | Approved | Beijing Genetech Pharmaceutical Co Ltd | 博固泰 | Mainland China | Osteoporosis | Beijing Genetech Pharmaceutical Co Ltd | 2024-01-16 | Osteoporosis; Osteoporosis, Postmenopausal | Details | |
| Teriparatide biosimilar (Virchow/Sun) | rhPTH (1-34) (Virchow/Sun) | Approved | Sun Pharmaceutical Industries Ltd, Virchow Group | Bonista, Osteotide | EU | Osteoporosis; Osteoporosis, Postmenopausal | Sun Pharmaceutical Industries (Europe) Bv | Osteoporosis; Osteoporosis, Postmenopausal | Details | |
| Teriparatide biosimilar (CinnaGen) | rhPTH (1-34) (CinnaGen) | Approved | Cinnagen | CinnoPar | Iran | Osteoporosis | Cinnagen | 2013-01-01 | Osteoporosis | Details |
| Teriparatide biosimilar (Zydus Cadila) | rhPTH (1-34) (Zydus Cadila) | Approved | Zydus Cadila | India | Osteoporosis | Zydus Cadila | 2014-01-01 | Osteoporosis | Details | |
| Palopegteriparatide | ACP-014; ACP014; ACP 014 | Approved | Ascendis Pharma | TransCon, YORVIPATH | EU | Hypoparathyroidism | Ascendis Pharma, Ascendis Pharma Bone Diseases As | 2023-11-17 | Hypoparathyroidism | Details |
| Teriparatide biosimilar (Biosidus) | rhPTH (1-34) (Biosidus) | Approved | Biosidus | Osteofortil | Argentina | Osteoporosis | Biosidus | 2012-08-01 | Osteoporosis | Details |
| Abaloparatide | ITM-058; BA-058-SC; BA-058-TD; BIM-44058; BA-058 | Approved | Ipsen | ELADYNOS, Tymlos | United States | Osteoporosis, Postmenopausal | Radius Health Inc | 2017-04-28 | Osteoporotic Fractures; Osteoporosis; Osteoporosis, Postmenopausal; Intervertebral Disc Degeneration; Fractures, Bone | Details |
| Teriparatide biosimilar (Amega Biotech) | rhPTH (1-34) (Amega Biotech) | Approved | Amega Biotech | Osteoporosis | Details | |||||
| Teriparatide biosimilar (Pfenex) | rhPTH (1-34) (Pfenex); PF-708 | Approved | Pfenex Biopharmaceuticals Inc | Bonsity, Qutavina | United States | Osteoporosis, Postmenopausal; Glucocorticoid-induced osteoporosis; Osteoporosis | Alvogen | 2019-10-04 | Osteoporosis; Osteoporosis, Postmenopausal; Glucocorticoid-induced osteoporosis | Details |
| Teriparatide | MN-10T; LY 333334; hPTH-1-34; EB-613 | Approved | Eli Lilly And Company | Forteo, Forsteo, 复泰奥 | United States | Osteoporosis | Eli Lilly And Company | 2002-11-26 | Ankle Fractures; Chronic Kidney Disease-Mineral and Bone Disorder; Anorexia Nervosa; Hypoparathyroidism; Periodontitis; Fractures, Bone; Osteoarthritis, Knee; Back Pain; Spinal Fractures; Humeral Fractures; Femoral Neck Fractures; Cartilage Diseases; Hypocalcemia; Colles' Fracture; Osteoporosis, Postmenopausal; Menopausal symptoms; Spinal Cord Injuries; Bone Diseases, Metabolic; Osteoporosis; Alveolar Bone Loss; Hyperparathyroidism, Secondary; Drug-Related Side Effects and Adverse Reactions | Details |
| Parathyroid hormone (Shire) | NPSP-795; rhPTH (1-84); rhPTH-1-84; PTH (1-84); SB-423562; PTH 1-84; NPSP-558; SHP-635; SHP-634; ALX-111; 423562; EBP-05; EB-612 | Approved | Shire | Natpara, Preotact, Natpar | United States | Hypoparathyroidism | Nps Pharmaceuticals, Inc | 2006-04-24 | Osteoporosis; Bone Diseases, Endocrine; Osteoporosis, Postmenopausal; Hypocalcemia; Psoriasis; Hypoparathyroidism; Hyperparathyroidism | Details |
| Teriparatide biosimilar (Intas Pharmaceuticals) | rhPTH (1-34) (Intas Pharmaceuticals); INTG8; INTG-8 | Approved | Intas Biopharmaceuticals | Terifrac | India | Osteoporosis | Intas Biopharmaceuticals | 2010-12-01 | Osteoporosis | Details |
| Teriparatide biosimilar (Gedeon Richter) | rhPTH (1-34) (Gedeon Richter) | Approved | Gedeon Richter Plc | Terrosa | EU | Osteoporosis | Gedeon Richter Plc | 2017-01-04 | Osteoporosis; Fractures, Stress | Details |
| Teriparatide biosimilar (Salubris) | SAL056; G56-W1; SAL-001 | Approved | Shenzhen Salubris Pharmaceuticals Co Ltd | 欣复泰 | Mainland China | Osteoporosis, Postmenopausal | Suzhou Genemen Biotech Co Ltd | 2019-09-29 | Osteoporosis, Postmenopausal | Details |
| Teriparatide biosimilar (USV) | Approved | Usv Ltd | Osteoporosis | Details | ||||||
| Teriparatide biosimilar (Strides Pharma) | SBL-001 | Approved | Strides Pharma | Kauliv | EU | Osteoporosis; Osteoporosis, Postmenopausal | Strides Pharma (Cyprus) Ltd | 2023-01-12 | Osteoporosis; Osteoporosis, Postmenopausal | Details |
| Teriparatide biosimilar (STADA Arzneimittel AG) | rhPTH (1-34) (STADA Arzneimittel AG) | Approved | Stada Arzneimittel Ag | Movymia | EU | Osteoporosis | Stada Arzneimittel Ag | 2017-01-11 | Osteoporosis | Details |
| Recombinant parathyroid hormone biosimilar(Chongqing Fujin Biological Medicine) | Approved | Chongqing Fujin Biological Medicine Co Ltd | Details | |||||||
| Recombinant Human Parathyroid Hormone (1-34), Shanghai Fosunpharma | Approved | Shanghai Fosun Pharmaceutical (Group) Co Ltd | Details | |||||||
| Teriparatide acetate | hPTH (1-34) acet. H2O; AK-159; MN-10-T | Approved | Union Square Ventures Llc | Parathar, Teribone | Japan | Osteoporosis; Hypocalcemia | null | 1987-03-31 | Osteoporosis; Hypocalcemia | Details |
| Teriparatide biosimilar (Theramex) | Approved | Theramex Ireland Ltd | Livogiva | EU | Osteoporosis | Theramex Ireland Ltd | 2020-08-27 | Osteoporosis | Details | |
| Teriparatide biosimilar (Shanghai United Cell ) | Approved | Shanghai United Cell Biotechnology Co Ltd | Mainland China | Osteoporosis | Shanghai United Cell Biotechnology Co Ltd | 2017-03-22 | Osteoporosis | Details | ||
| Teriparatide biosimilar (Beijing Bokangjian Gene) | Approved | Beijing Genetech Pharmaceutical Co Ltd | 博固泰 | Mainland China | Osteoporosis | Beijing Genetech Pharmaceutical Co Ltd | 2024-01-16 | Osteoporosis; Osteoporosis, Postmenopausal | Details | |
| Teriparatide biosimilar (Virchow/Sun) | rhPTH (1-34) (Virchow/Sun) | Approved | Sun Pharmaceutical Industries Ltd, Virchow Group | Bonista, Osteotide | EU | Osteoporosis; Osteoporosis, Postmenopausal | Sun Pharmaceutical Industries (Europe) Bv | Osteoporosis; Osteoporosis, Postmenopausal | Details | |
| Teriparatide biosimilar (CinnaGen) | rhPTH (1-34) (CinnaGen) | Approved | Cinnagen | CinnoPar | Iran | Osteoporosis | Cinnagen | 2013-01-01 | Osteoporosis | Details |
| Teriparatide biosimilar (Zydus Cadila) | rhPTH (1-34) (Zydus Cadila) | Approved | Zydus Cadila | India | Osteoporosis | Zydus Cadila | 2014-01-01 | Osteoporosis | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Teriparatide biosimilar (National Institute of Dental and Craniofacial Research) | rhPTH (1-34) (National Institute of Dental and Craniofacial Research) | Phase 3 Clinical | National Institute Of Dental And Craniofacial Research | DiGeorge Syndrome; Hypothyroidism; Hypoparathyroidism | Details |
| Teriparatide biosimilar (Shanghai CAS Shenglongda Biotech) | Phase 3 Clinical | Shanghai Zhongke Shenglongda Biotechnology (Group) Co Ltd | Osteoporosis, Postmenopausal | Details | |
| Parathyroid hormone 1-84 (Kerun Biopharmaceutical) | Phase 3 Clinical | Chongqing Kerun Biologic Pharmaceutical Development Co Ltd | Osteoporosis, Postmenopausal; Hypoparathyroidism | Details | |
| Eneboparatide | AZP-3601 | Phase 3 Clinical | Harvard Medical School, Massachusetts General Hospital | Endocrine System Diseases; Parathyroid Diseases; Hypoparathyroidism | Details |
| Teriparatide transdermal (Corium International) | Phase 2 Clinical | Corium International Inc | Osteoporosis | Details | |
| Teriparatide transdermal (Zosano Pharma) | B-106; B-104; ZP-PTH; ThPTH (1-34); TH-0229 | Phase 2 Clinical | Zosano Pharma Corp | Osteoporosis; Osteoporosis, Postmenopausal | Details |
| KUR-113 | KUR-113; I-040202; Fibrin-PTH | Phase 2 Clinical | Kuros Biosciences | Spinal Diseases; Intervertebral Disc Degeneration; Wounds and Injuries | Details |
| T3-sulfate (Bracco SpA) | Phase 2 Clinical | Bracco Spa | Hypothyroidism | Details | |
| Recombinant Human Parathyroid Hormone (1-34)(Shanghai Celgen Biopharmaceuticals) | Phase 2 Clinical | Shanghai Celgen Bio-Pharmaceutical Co Ltd | Osteoporosis | Details | |
| KUR-111 | I-0401; KUR-111 | Phase 2 Clinical | Kuros Biosciences | Fractures, Bone | Details |
| MT-1013 | MT-1013 | Phase 2 Clinical | Shaanxi Micot Technology Co Ltd | Hyperparathyroidism, Secondary | Details |
| Teriparatide biosimilar (Shanghai Fudan-Zhangjiang Bio-Pharmaceutical) | Phase 1 Clinical | Shanghai Fudan-Zhangjiang Bio-Pharmaceutical Co Ltd | Osteoporosis | Details | |
| PCO-371 | PCO-371 | Phase 1 Clinical | Chugai Pharmaceutical Co Ltd | Hypoparathyroidism | Details |
| Hemoparatide | hPTH-137 | Phase 1 Clinical | Pharis Biotec | Psoriasis | Details |
| EXT-608 | EXT-608 | Phase 1 Clinical | Extend Biosciences Inc | Details | |
| Teriparatide biosimilar(Hybio Pharmaceutical) | Phase 1 Clinical | Hybio Pharmaceutical Co Ltd | Osteoporosis, Postmenopausal | Details | |
| RT-102(Rani) | RT-102 | Phase 1 Clinical | Rani Therapeutics Holdings Inc | Osteoporosis | Details |
| MBX-2109 | MBX-2109 | Phase 1 Clinical | MBX Biosciences Inc | Details | |
| Parathyroid hormone 1-84(Sichuan Luzhou Buchang Biopharmaceutical) | Phase 1 Clinical | Sichuan Luzhou Buchang Biopharmaceutical Co Ltd | Hypocalcemia | Details | |
| MT-1009 | MT-1009 | Phase 1 Clinical | Shaanxi Micot Technology Co Ltd | Osteoporosis; Glucocorticoid-induced osteoporosis | Details |
| BMD-1221 | BMD-1221 | Clinical | University Of Arkansas System | Fractures, Bone | Details |
| Teriparatide biosimilar (National Institute of Dental and Craniofacial Research) | rhPTH (1-34) (National Institute of Dental and Craniofacial Research) | Phase 3 Clinical | National Institute Of Dental And Craniofacial Research | DiGeorge Syndrome; Hypothyroidism; Hypoparathyroidism | Details |
| Teriparatide biosimilar (Shanghai CAS Shenglongda Biotech) | Phase 3 Clinical | Shanghai Zhongke Shenglongda Biotechnology (Group) Co Ltd | Osteoporosis, Postmenopausal | Details | |
| Parathyroid hormone 1-84 (Kerun Biopharmaceutical) | Phase 3 Clinical | Chongqing Kerun Biologic Pharmaceutical Development Co Ltd | Osteoporosis, Postmenopausal; Hypoparathyroidism | Details | |
| Eneboparatide | AZP-3601 | Phase 3 Clinical | Harvard Medical School, Massachusetts General Hospital | Endocrine System Diseases; Parathyroid Diseases; Hypoparathyroidism | Details |
| Teriparatide transdermal (Corium International) | Phase 2 Clinical | Corium International Inc | Osteoporosis | Details | |
| Teriparatide transdermal (Zosano Pharma) | B-106; B-104; ZP-PTH; ThPTH (1-34); TH-0229 | Phase 2 Clinical | Zosano Pharma Corp | Osteoporosis; Osteoporosis, Postmenopausal | Details |
| KUR-113 | KUR-113; I-040202; Fibrin-PTH | Phase 2 Clinical | Kuros Biosciences | Spinal Diseases; Intervertebral Disc Degeneration; Wounds and Injuries | Details |
| T3-sulfate (Bracco SpA) | Phase 2 Clinical | Bracco Spa | Hypothyroidism | Details | |
| Recombinant Human Parathyroid Hormone (1-34)(Shanghai Celgen Biopharmaceuticals) | Phase 2 Clinical | Shanghai Celgen Bio-Pharmaceutical Co Ltd | Osteoporosis | Details | |
| KUR-111 | I-0401; KUR-111 | Phase 2 Clinical | Kuros Biosciences | Fractures, Bone | Details |
| MT-1013 | MT-1013 | Phase 2 Clinical | Shaanxi Micot Technology Co Ltd | Hyperparathyroidism, Secondary | Details |
| Teriparatide biosimilar (Shanghai Fudan-Zhangjiang Bio-Pharmaceutical) | Phase 1 Clinical | Shanghai Fudan-Zhangjiang Bio-Pharmaceutical Co Ltd | Osteoporosis | Details | |
| PCO-371 | PCO-371 | Phase 1 Clinical | Chugai Pharmaceutical Co Ltd | Hypoparathyroidism | Details |
| Hemoparatide | hPTH-137 | Phase 1 Clinical | Pharis Biotec | Psoriasis | Details |
| EXT-608 | EXT-608 | Phase 1 Clinical | Extend Biosciences Inc | Details | |
| Teriparatide biosimilar(Hybio Pharmaceutical) | Phase 1 Clinical | Hybio Pharmaceutical Co Ltd | Osteoporosis, Postmenopausal | Details | |
| RT-102(Rani) | RT-102 | Phase 1 Clinical | Rani Therapeutics Holdings Inc | Osteoporosis | Details |
| MBX-2109 | MBX-2109 | Phase 1 Clinical | MBX Biosciences Inc | Details | |
| Parathyroid hormone 1-84(Sichuan Luzhou Buchang Biopharmaceutical) | Phase 1 Clinical | Sichuan Luzhou Buchang Biopharmaceutical Co Ltd | Hypocalcemia | Details | |
| MT-1009 | MT-1009 | Phase 1 Clinical | Shaanxi Micot Technology Co Ltd | Osteoporosis; Glucocorticoid-induced osteoporosis | Details |
| BMD-1221 | BMD-1221 | Clinical | University Of Arkansas System | Fractures, Bone | Details |
This web search service is supported by Google Inc.
