 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
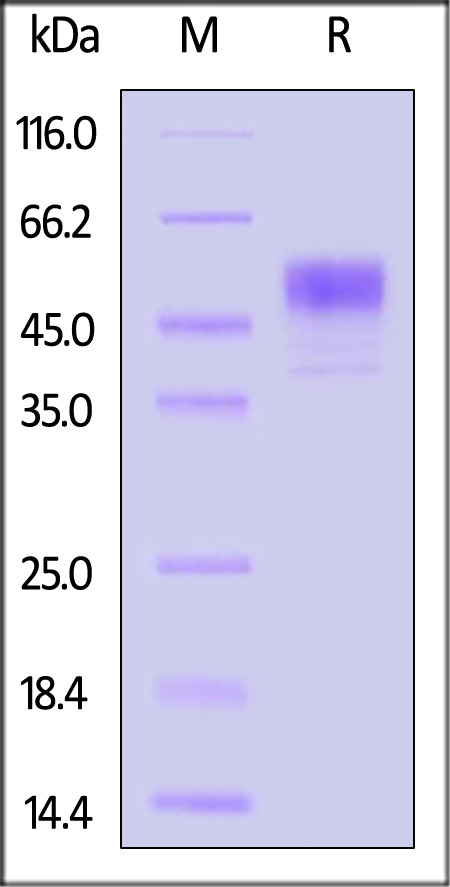
| IL3-H52G3 | Human | Human IL-13 Protein, GST Tag |  |
|
|
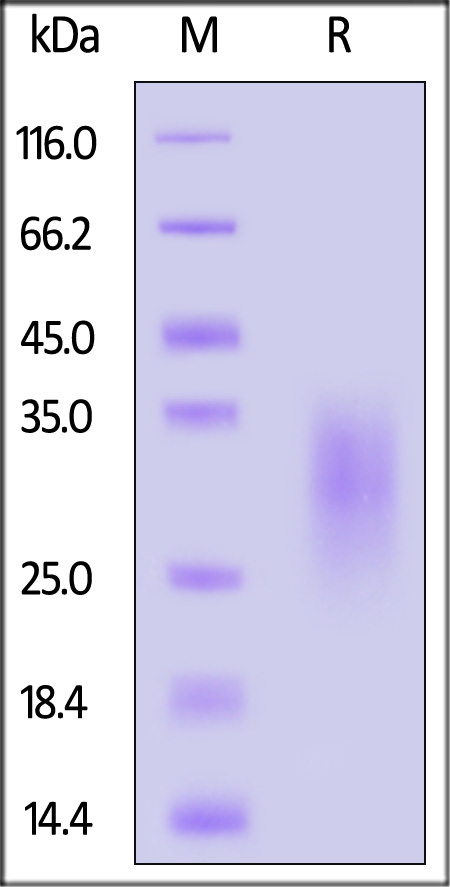
| IL3-C5249 | Cynomolgus | Cynomolgus IL-13 Protein, His Tag (MALS verified) |  |
|
|
| IL3-M5249 | Mouse | Mouse IL-13 Protein, His Tag |  |
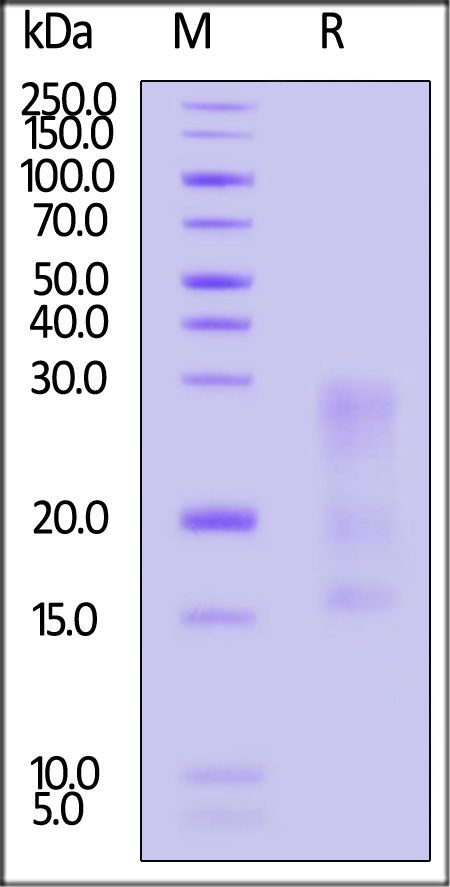
|
|
| IL3-C52H4 | Canine | Canine IL-13 Protein, His Tag |  |
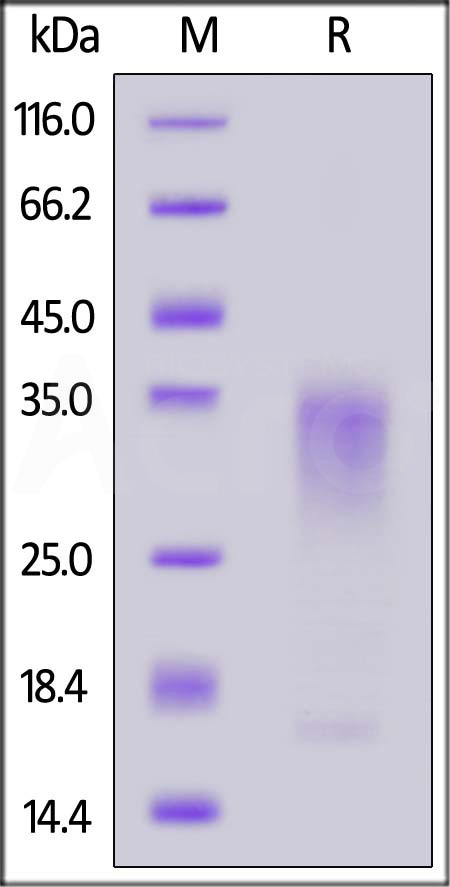
|
|
| IL3-H82E5 | Human | Biotinylated Human IL-13 Protein, His,Avitag™ (MALS verified) | 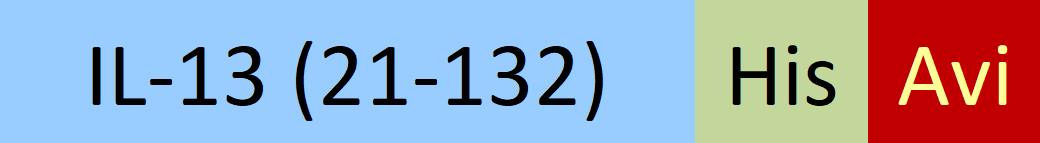 |
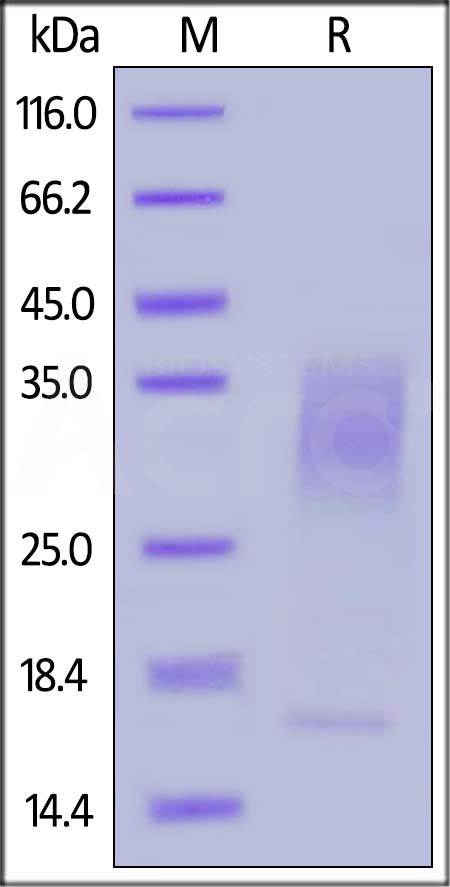
|
|
| IL3-H5256 | Human | Human IL-13 Protein, Fc Tag |  |

|
|
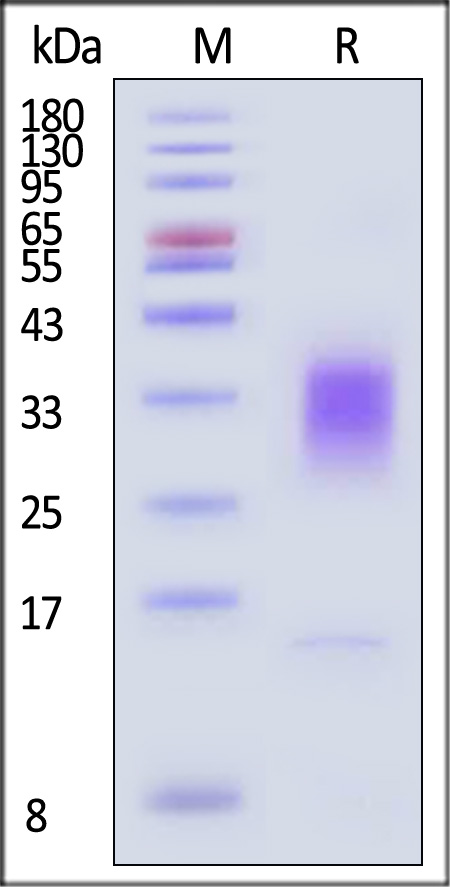
| IL3-H52H4 | Human | Human IL-13 Protein, His Tag | 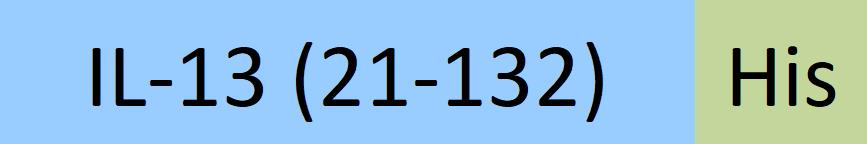 |
|
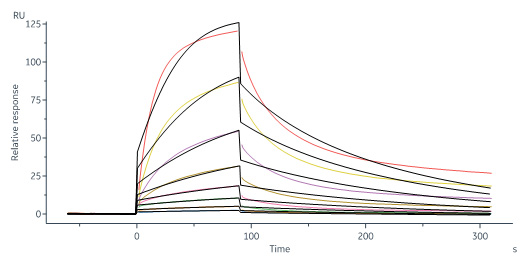
Biotinylated Human IL-13 R alpha 1 Protein, His,Avitag (Cat. No. IL1-H82E8) captured on Biotin CAP-Series S Sensor Chip can bind Human IL-13 Protein, His Tag (Cat. No. IL3-H52H4) with an affinity constant of 34.4 nM as determined in a SPR assay (Biacore 8K) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Berdazimer sodium-SB-206 (Novan) | NVN-1000-SB-206-Novan; MAP3-NONOate - SB-206; NVN1000-SB206; SB-206; SB-019; NI-MC101 | Approved | Zelsuvmi, ZELSUVMI, KINSOLUSTM | United States | Molluscum Contagiosum | Lnhc Inc | 2024-01-05 | Acne Vulgaris; Tinea Pedis; Psoriasis; Molluscum Contagiosum; Condylomata Acuminata; Dermatitis, Atopic | Details | |
| Lebrikizumab | RO-4909832; PRO-301444; DRM-06; Anti-IL-13; TNX-650; RG-3637; RO-5490255; MILR-1444A; R-3637; DRM 06; LY 3650150; MILR1444A; MILR1444Ab; RG 3637; RO 5490255; TNX 650 | Approved | Tanox Inc | EBGLYSS | EU | Dermatitis, Atopic | Almirall Sa | 2023-11-16 | Idiopathic Pulmonary Fibrosis; Hodgkin Disease; Skin Diseases; Dermatitis; Asthma; Sinusitis; Alzheimer Disease; Pulmonary Disease, Chronic Obstructive; Dermatitis, Atopic; Eczema; Skin Diseases, Genetic | Details |
| Tralokinumab | BAK-502G9; CAT-354 | Approved | Medimmune Llc | Adtralza, ADBRY | EU | Dermatitis, Atopic | Leo Pharma A/S | 2021-06-17 | Idiopathic Pulmonary Fibrosis; Colitis, Ulcerative; Asthma; Dermatitis, Atopic; Eczema | Details |
| Berdazimer sodium-SB-206 (Novan) | NVN-1000-SB-206-Novan; MAP3-NONOate - SB-206; NVN1000-SB206; SB-206; SB-019; NI-MC101 | Approved | Zelsuvmi, ZELSUVMI, KINSOLUSTM | United States | Molluscum Contagiosum | Lnhc Inc | 2024-01-05 | Acne Vulgaris; Tinea Pedis; Psoriasis; Molluscum Contagiosum; Condylomata Acuminata; Dermatitis, Atopic | Details | |
| Lebrikizumab | RO-4909832; PRO-301444; DRM-06; Anti-IL-13; TNX-650; RG-3637; RO-5490255; MILR-1444A; R-3637; DRM 06; LY 3650150; MILR1444A; MILR1444Ab; RG 3637; RO 5490255; TNX 650 | Approved | Tanox Inc | EBGLYSS | EU | Dermatitis, Atopic | Almirall Sa | 2023-11-16 | Idiopathic Pulmonary Fibrosis; Hodgkin Disease; Skin Diseases; Dermatitis; Asthma; Sinusitis; Alzheimer Disease; Pulmonary Disease, Chronic Obstructive; Dermatitis, Atopic; Eczema; Skin Diseases, Genetic | Details |
| Tralokinumab | BAK-502G9; CAT-354 | Approved | Medimmune Llc | Adtralza, ADBRY | EU | Dermatitis, Atopic | Leo Pharma A/S | 2021-06-17 | Idiopathic Pulmonary Fibrosis; Colitis, Ulcerative; Asthma; Dermatitis, Atopic; Eczema | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Berdazimer sodium-SB-204 (Novan) | MAP3-NONOate-SB-204; NVN1000-SB204; SB-204 | Phase 3 Clinical | University Of North Carolina At Chapel Hill | Acne Vulgaris | Details |
| Cendakimab | RPC-4046; ABT-308; CC-93538 | Phase 3 Clinical | Abbott Laboratories | Eosinophilic gastroenteritis (EG); Eosinophilic Esophagitis; Asthma; Dermatitis, Atopic; Eczema | Details |
| Eblasakimab | MK-6105; ASLAN-004; CSL-334 | Phase 2 Clinical | Csl Ltd, Merck Sharp & Dohme Corp | Dermatitis, Atopic; Hypersensitivity | Details |
| Dectrekumab/VAK-694 | QBX-258 | Phase 2 Clinical | Novartis Pharma Ag | Lymphedema; Asthma | Details |
| PF-07264660 | PF-07264660 | Phase 2 Clinical | Pfizer Inc | Dermatitis, Atopic | Details |
| PF-07275315 | PF-07275315 | Phase 2 Clinical | Pfizer Inc | Dermatitis, Atopic | Details |
| Lunsekimig | SAR-443765 | Phase 2 Clinical | Sanofi | Asthma; Inflammation | Details |
| APG-777 | APG777; APG-777; PR004; PR-004 | Phase 1 Clinical | Apogee Therapeutics Inc | Asthma; Dermatitis, Atopic | Details |
| SAR-443726 | SAR-443726 | Phase 1 Clinical | Sanofi | Dermatitis, Atopic | Details |
| BD-9 | BD-9 | Biolojic Design Inc | Details | ||
| Berdazimer sodium-SB-204 (Novan) | MAP3-NONOate-SB-204; NVN1000-SB204; SB-204 | Phase 3 Clinical | University Of North Carolina At Chapel Hill | Acne Vulgaris | Details |
| Cendakimab | RPC-4046; ABT-308; CC-93538 | Phase 3 Clinical | Abbott Laboratories | Eosinophilic gastroenteritis (EG); Eosinophilic Esophagitis; Asthma; Dermatitis, Atopic; Eczema | Details |
| Eblasakimab | MK-6105; ASLAN-004; CSL-334 | Phase 2 Clinical | Csl Ltd, Merck Sharp & Dohme Corp | Dermatitis, Atopic; Hypersensitivity | Details |
| Dectrekumab/VAK-694 | QBX-258 | Phase 2 Clinical | Novartis Pharma Ag | Lymphedema; Asthma | Details |
| PF-07264660 | PF-07264660 | Phase 2 Clinical | Pfizer Inc | Dermatitis, Atopic | Details |
| PF-07275315 | PF-07275315 | Phase 2 Clinical | Pfizer Inc | Dermatitis, Atopic | Details |
| Lunsekimig | SAR-443765 | Phase 2 Clinical | Sanofi | Asthma; Inflammation | Details |
| APG-777 | APG777; APG-777; PR004; PR-004 | Phase 1 Clinical | Apogee Therapeutics Inc | Asthma; Dermatitis, Atopic | Details |
| SAR-443726 | SAR-443726 | Phase 1 Clinical | Sanofi | Dermatitis, Atopic | Details |
| BD-9 | BD-9 | Biolojic Design Inc | Details |
This web search service is supported by Google Inc.
