 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
| TL3-R5253 | Rhesus macaque | Rhesus macaque TLR3 / CD283 Protein, Fc Tag (MALS verified) |  |
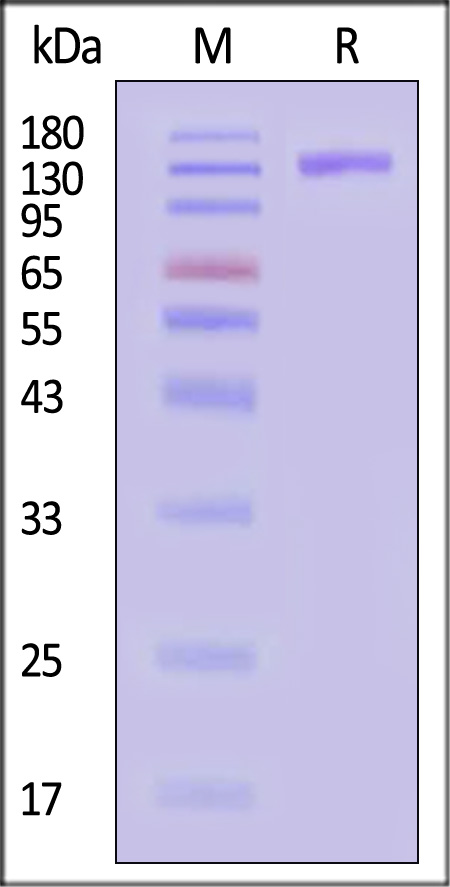
|
|
| TL3-M5255 | Mouse | Mouse TLR3 / CD283 Protein, Fc Tag (MALS verified) |  |
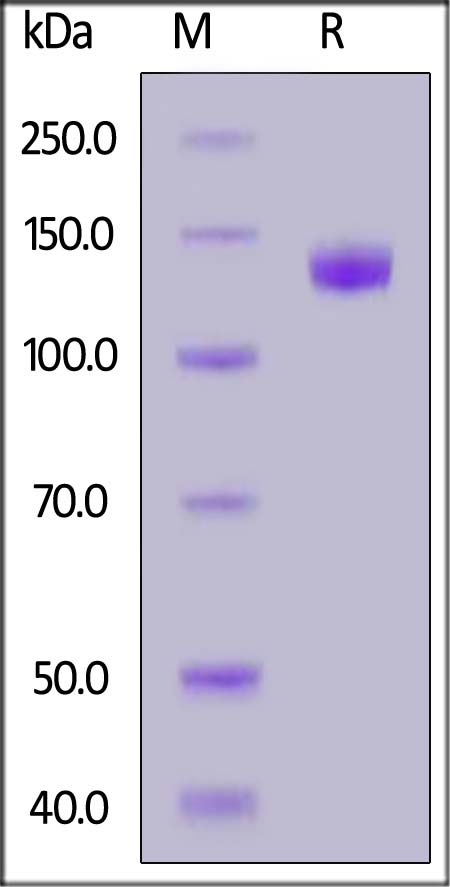
|
|
| TL3-H5253 | Human | Human TLR3 Protein, Fc Tag (MALS verified) |  |
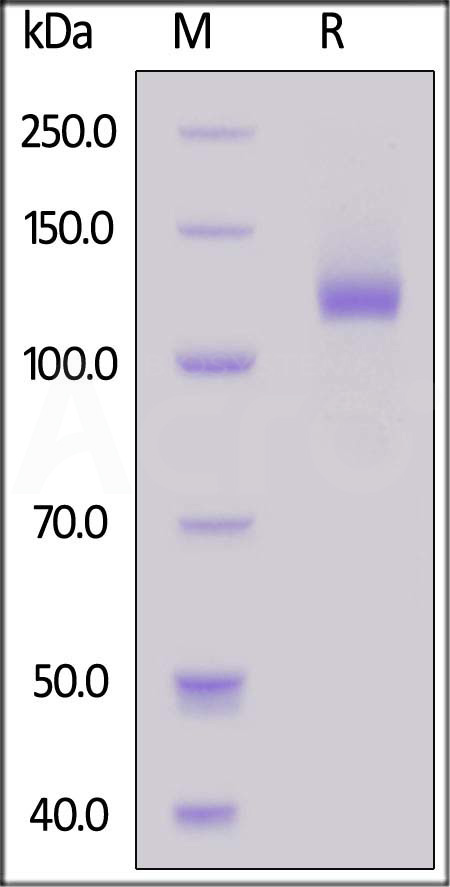
|
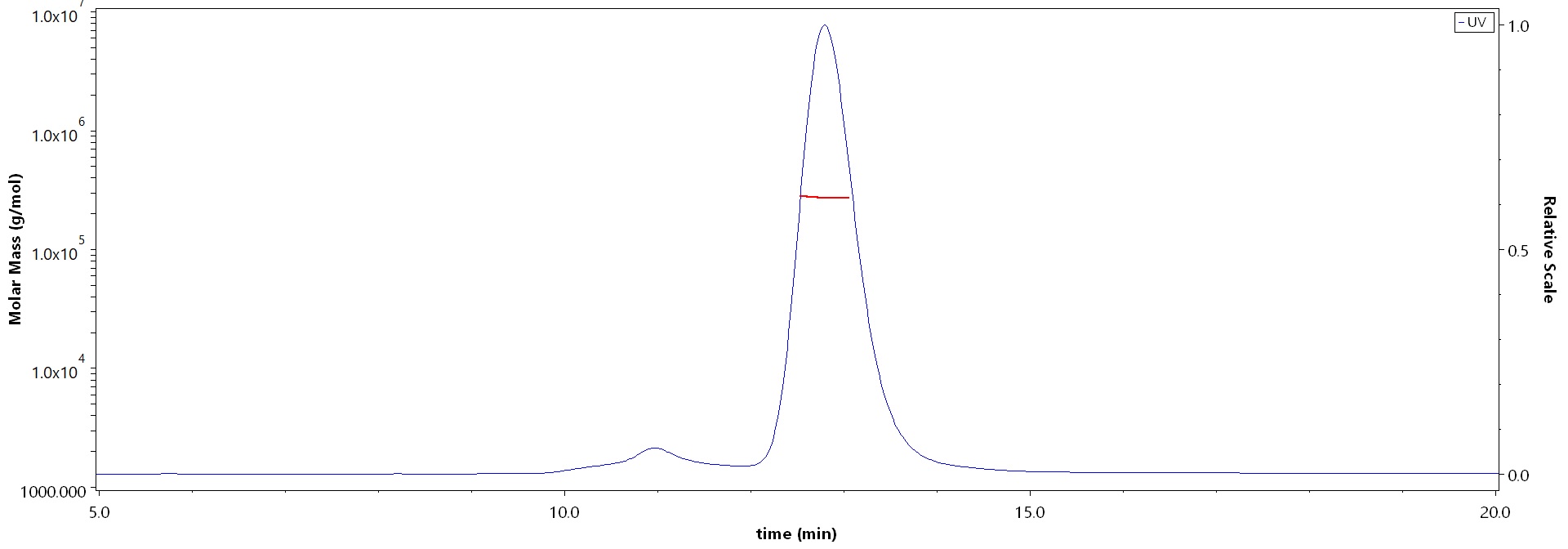
The purity of Rhesus macaque TLR3 Protein, Fc Tag (Cat. No. TL3-R5253) is more than 90% and the molecular weight of this protein is around 260-290 kDa verified by SEC-MALS.

The purity of Human TLR3, Fc Tag (Cat. No. TL3-M5255) is more than 90% and the molecular weight of this protein is around 235-287 kDa verified by SEC-MALS.
Please contact us via TechSupport@acrobiosystems.com if you have any question on this product.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Rintatolimod | AMP-516; AMP-518 | Approved | Hemispherx Biopharma Inc | Rintamod, Ampligen | Argentina | Fatigue Syndrome, Chronic | Hemispherx Biopharma Inc | 2017-01-01 | HIV Infections; Ovarian Neoplasms; Pancreatic Neoplasms; Mesothelioma; Influenza, Human; Breast Neoplasms; Post-Acute COVID-19 Syndrome; Peritoneal Neoplasms; Colorectal Neoplasms; Fatigue Syndrome, Chronic; Fallopian Tube Neoplasms; HIV Seropositivity; Breast Neoplasms, Male | Details |
| Rintatolimod | AMP-516; AMP-518 | Approved | Hemispherx Biopharma Inc | Rintamod, Ampligen | Argentina | Fatigue Syndrome, Chronic | Hemispherx Biopharma Inc | 2017-01-01 | HIV Infections; Ovarian Neoplasms; Pancreatic Neoplasms; Mesothelioma; Influenza, Human; Breast Neoplasms; Post-Acute COVID-19 Syndrome; Peritoneal Neoplasms; Colorectal Neoplasms; Fatigue Syndrome, Chronic; Fallopian Tube Neoplasms; HIV Seropositivity; Breast Neoplasms, Male | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Monovalent norovirus Vaccine (Vaxart) | VXA-G1-1-NN; VXA-G1.1-NN | Phase 2 Clinical | Vaxart Inc | Norovirus Infections | Details |
| TR-987 | Z-101; GLYC-101; MG-3601 | Phase 2 Clinical | Kazia Therapeutics | Wounds and Injuries; Varicose Ulcer | Details |
| Poly ICLC (Oncovir) | Phase 2 Clinical | National Institutes Of Health, Oncovir Inc | Prostatic Neoplasms; Melanoma; Carcinoma, Non-Small-Cell Lung; Lymphoma, T-Cell; Glioma; Carcinoma, Squamous Cell; Colorectal Neoplasms; Breast Neoplasms; Carcinoma, Intraductal, Noninfiltrating; Lymphoma, B-Cell; Sarcoma; Osteosarcoma; Mesothelioma; Coronavirus Disease 2019 (COVID-19); Pancreatic Neoplasms; Glioblastoma; Carcinoma, Merkel Cell | Details | |
| BO-112 | BO-112 | Phase 2 Clinical | Stomach Neoplasms; Esophageal Neoplasms; Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Melanoma; Carcinoma, Hepatocellular | Details | |
| Monovalent norovirus Vaccine (Vaxart)/VXA-G2-4-NS | VXA-NVV-201 | Phase 2 Clinical | Vaxart Inc | Norovirus Infections | Details |
| CNTO-5 | CNTO-5 | Phase 1 Clinical | Morphosys Ag | Inflammation | Details |
| PGV-001 | PGV-001 | Phase 1 Clinical | Genentech Inc, Icahn School Of Medicine At Mount Sinai | Neoplasms; Urologic Neoplasms | Details |
| Monovalent norovirus Vaccine (Vaxart) | VXA-G1-1-NN; VXA-G1.1-NN | Phase 2 Clinical | Vaxart Inc | Norovirus Infections | Details |
| TR-987 | Z-101; GLYC-101; MG-3601 | Phase 2 Clinical | Kazia Therapeutics | Wounds and Injuries; Varicose Ulcer | Details |
| Poly ICLC (Oncovir) | Phase 2 Clinical | National Institutes Of Health, Oncovir Inc | Prostatic Neoplasms; Melanoma; Carcinoma, Non-Small-Cell Lung; Lymphoma, T-Cell; Glioma; Carcinoma, Squamous Cell; Colorectal Neoplasms; Breast Neoplasms; Carcinoma, Intraductal, Noninfiltrating; Lymphoma, B-Cell; Sarcoma; Osteosarcoma; Mesothelioma; Coronavirus Disease 2019 (COVID-19); Pancreatic Neoplasms; Glioblastoma; Carcinoma, Merkel Cell | Details | |
| BO-112 | BO-112 | Phase 2 Clinical | Stomach Neoplasms; Esophageal Neoplasms; Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Melanoma; Carcinoma, Hepatocellular | Details | |
| Monovalent norovirus Vaccine (Vaxart)/VXA-G2-4-NS | VXA-NVV-201 | Phase 2 Clinical | Vaxart Inc | Norovirus Infections | Details |
| CNTO-5 | CNTO-5 | Phase 1 Clinical | Morphosys Ag | Inflammation | Details |
| PGV-001 | PGV-001 | Phase 1 Clinical | Genentech Inc, Icahn School Of Medicine At Mount Sinai | Neoplasms; Urologic Neoplasms | Details |
This web search service is supported by Google Inc.
