 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
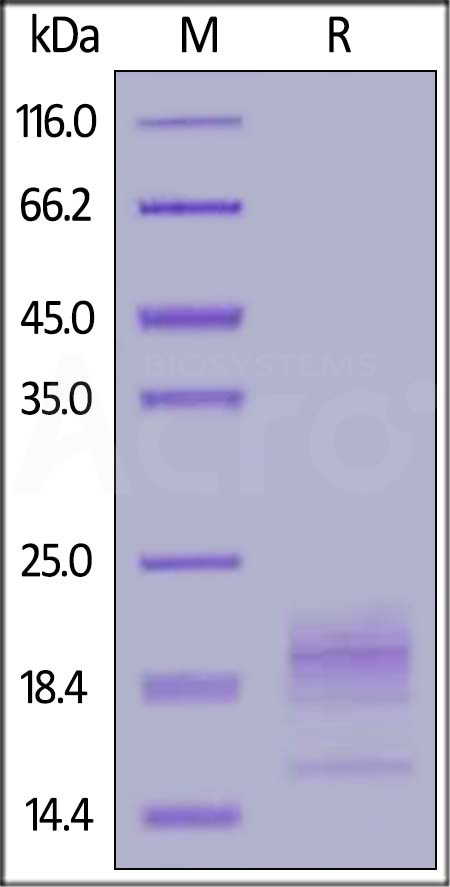
| CD2-H82E8 | Human | Biotinylated Human CD52 Protein, His,Avitag™ |  |
|
|
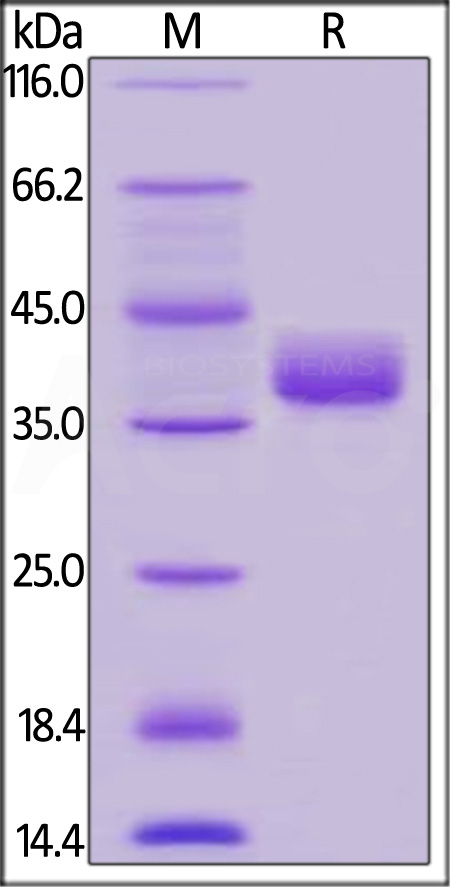
| CD2-H525a | Human | Human CD52 Protein, Fc Tag |  |
|
|
| CD2-H82F3 | Human | Biotinylated Human CD52 Protein, Fc,Avitag™ |  |
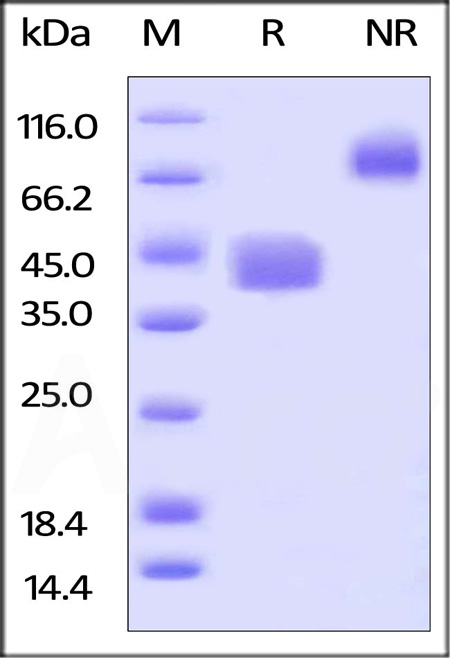
|
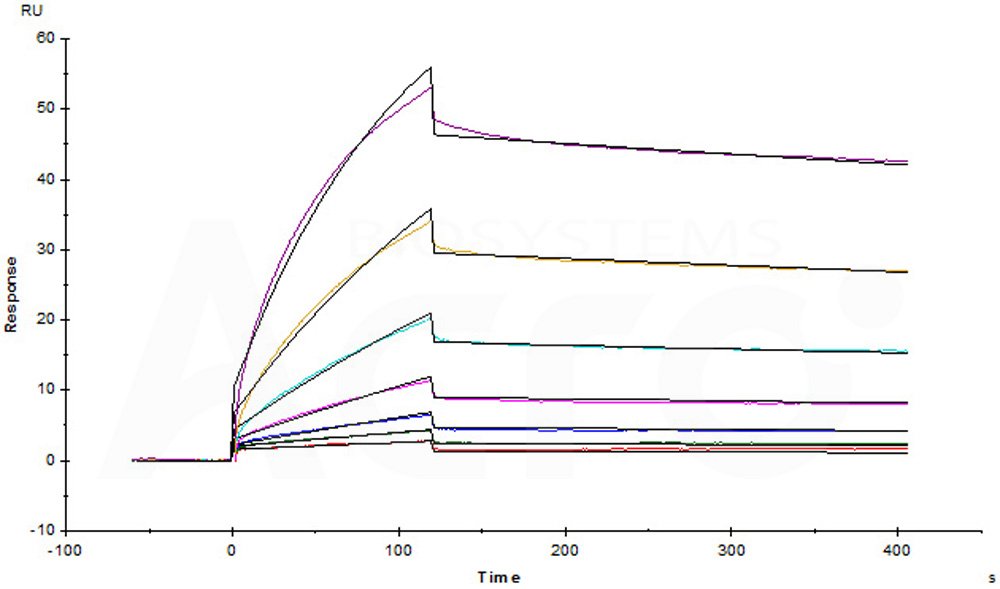
Anti-Human CD52 MAb (Human IgG1) captured on CM5 chip via anti-human IgG Fc antibodies surface can bind Biotinylated Human CD52, His,Avitag (Cat. No. CD2-H82E8) with an affinity constant of 71.8 nM as determined in a SPR assay (Biacore T200) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Alemtuzumab | LDP-03; GZ-402673 | Approved | Genzyme Corp, Bayer AG | Campath, Lemtrada, MabCampath | United States | Multiple Sclerosis | Genzyme Corp | 2001-05-07 | Myositis, Inclusion Body; Leukemia, Lymphocytic, Chronic, B-Cell; Mycosis Fungoides; Lymphoma, Non-Hodgkin; Fallopian Tube Neoplasms; Leukemia, Myeloid, Acute; Waldenstrom Macroglobulinemia; Lymphoma; Leukemia, Prolymphocytic, T-Cell; Lymphoma, T-Cell, Cutaneous; Lymphoma, T-Cell; Lymphoproliferative Disorders; Peritoneal Neoplasms; Sezary Syndrome; Pancytopenia; Lymphoma, T-Cell, Peripheral; Multiple Sclerosis; Rejection of organ transplantation; Leukemia-Lymphoma, Adult T-Cell; Myelodysplastic Syndromes; Hodgkin Disease; Graft vs Host Disease; Kidney Diseases; Rejection of renal transplantation; Hematologic Neoplasms; Ovarian Neoplasms; Diabetes Mellitus, Type 1; Bone marrow transplant rejection; Leukemia; Multiple Sclerosis, Relapsing-Remitting | Details |
| Alemtuzumab | LDP-03; GZ-402673 | Approved | Genzyme Corp, Bayer AG | Campath, Lemtrada, MabCampath | United States | Multiple Sclerosis | Genzyme Corp | 2001-05-07 | Myositis, Inclusion Body; Leukemia, Lymphocytic, Chronic, B-Cell; Mycosis Fungoides; Lymphoma, Non-Hodgkin; Fallopian Tube Neoplasms; Leukemia, Myeloid, Acute; Waldenstrom Macroglobulinemia; Lymphoma; Leukemia, Prolymphocytic, T-Cell; Lymphoma, T-Cell, Cutaneous; Lymphoma, T-Cell; Lymphoproliferative Disorders; Peritoneal Neoplasms; Sezary Syndrome; Pancytopenia; Lymphoma, T-Cell, Peripheral; Multiple Sclerosis; Rejection of organ transplantation; Leukemia-Lymphoma, Adult T-Cell; Myelodysplastic Syndromes; Hodgkin Disease; Graft vs Host Disease; Kidney Diseases; Rejection of renal transplantation; Hematologic Neoplasms; Ovarian Neoplasms; Diabetes Mellitus, Type 1; Bone marrow transplant rejection; Leukemia; Multiple Sclerosis, Relapsing-Remitting | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Alemtuzumab biosimilar (Zhangjiang Biotechnology) | Phase 2 Clinical | Shanghai Zhangjiang Biotechnology Co Ltd | Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| ALLO-647 | ALLO-647 | Phase 2 Clinical | Allogene Therapeutics Inc | Lymphoma, B-Cell; Carcinoma, Renal Cell; Multiple Myeloma; Lymphoma, Follicular; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Recombinant anti-CD52 humanized monoclonal antibody (Lanzhou Institute Of Biological Products/Shanghai Taiyin Biotechnology) | Phase 1 Clinical | Lanzhou Institute Of Biological Products Co Ltd, Shanghai Taiyin Biotechnology Co Ltd | Leukemia, B-Cell; Leukemia, Prolymphocytic, T-Cell; Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma, T-Cell; Leukemia, Lymphocytic, Chronic, B-Cell; Leukemia, Prolymphocytic | Details | |
| Alemtuzumab biosimilar(BioXpress) | BX-1523; BXT-1523 | Clinical | Bioxpress Therapeutics Sa | Multiple Sclerosis; Leukemia, B-Cell | Details |
| Alemtuzumab biosimilar (Zhangjiang Biotechnology) | Phase 2 Clinical | Shanghai Zhangjiang Biotechnology Co Ltd | Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| ALLO-647 | ALLO-647 | Phase 2 Clinical | Allogene Therapeutics Inc | Lymphoma, B-Cell; Carcinoma, Renal Cell; Multiple Myeloma; Lymphoma, Follicular; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Recombinant anti-CD52 humanized monoclonal antibody (Lanzhou Institute Of Biological Products/Shanghai Taiyin Biotechnology) | Phase 1 Clinical | Lanzhou Institute Of Biological Products Co Ltd, Shanghai Taiyin Biotechnology Co Ltd | Leukemia, B-Cell; Leukemia, Prolymphocytic, T-Cell; Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma, T-Cell; Leukemia, Lymphocytic, Chronic, B-Cell; Leukemia, Prolymphocytic | Details | |
| Alemtuzumab biosimilar(BioXpress) | BX-1523; BXT-1523 | Clinical | Bioxpress Therapeutics Sa | Multiple Sclerosis; Leukemia, B-Cell | Details |
This web search service is supported by Google Inc.
