 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Project Name | Modality | Therapeutic Area | Indications | Stage | Right Available |
| PD-1+VEGF bsAb | Bispecific antibody | Oncology/Cancer | Solid tumor | Phase I | Global (except China) |
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
| VE0-H52H3 | Human | Human VEGF110 Protein, His Tag (MALS verified) |  |
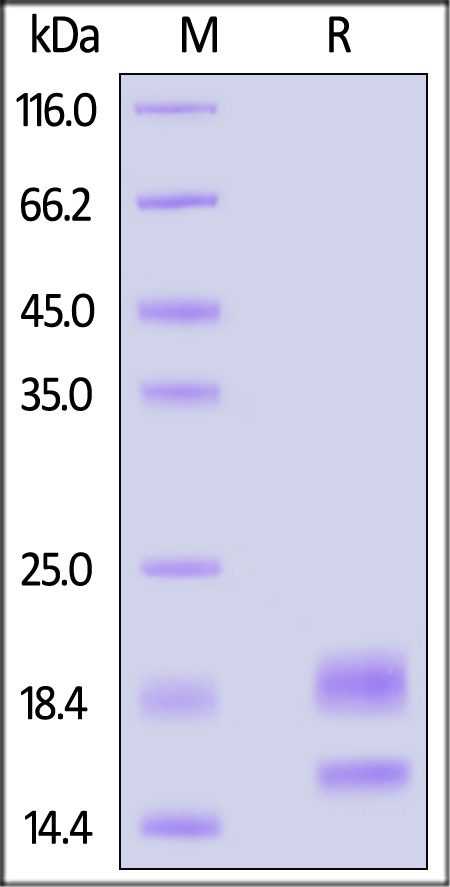
|
|
| VE0-H5212 | Human | Human VEGF110 Protein, premium grade |  |

|
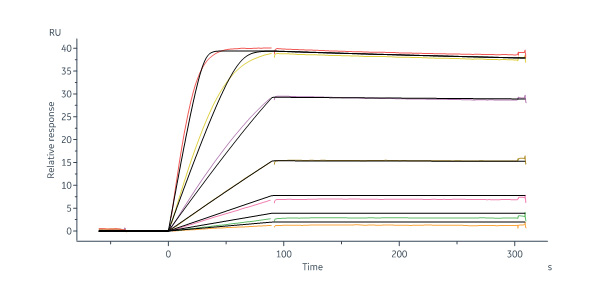
Human VEGFR1/R2 captured on Protein A Chip can bind Human VEGF110 Protein, His Tag (Cat. No. VE0-H52H3) with an affinity constant of 13.8 pM as determined in a SPR assay (Biacore 8K) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Bevacizumab biosimilar (Qilu Pharma) | QL-1101 | Approved | Qilu Pharmaceutical Co Ltd | 安可达 | Mainland China | Carcinoma, Non-Small-Cell Lung; Colorectal Neoplasms | Qilu Pharmaceutical Co Ltd | 2019-12-06 | Neoplasms; Colorectal Neoplasms; Carcinoma, Neuroendocrine; Carcinoma, Non-Small-Cell Lung | Details |
| Bevacizumab biosimilar (Innovent Biologics) | IBI-305; IBI305; IBI 305; CHS-305 | Approved | Innovent Biologics(Suzhou) Co Ltd | 达攸同, BYVASDA, Bevagen | Mainland China | Carcinoma, Non-Small-Cell Lung; Colorectal Neoplasms | Innovent Biologics(Suzhou) Co Ltd | 2020-06-17 | Ovarian Neoplasms; Glioblastoma; Carcinoma, Ovarian Epithelial; Triple Negative Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Uterine Cervical Neoplasms | Details |
| Bevacizumab biosimilar (Pfizer) | PF-6439535; PF-06439535 | Approved | Pfizer Inc | Zirabev | EU | Carcinoma, Renal Cell; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms; Fallopian Tube Neoplasms | Pfizer Europe Ma Eeig | 2019-02-14 | Ovarian Neoplasms; Carcinoma, Renal Cell; Glioblastoma; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Bevacizumab biosimilar (Elea) | Approved | Laboratorio Elea Phoenix Sa | Lumiere | Argentina | Macular Degeneration | Laboratorio Elea Phoenix Sa | 2018-04-26 | Macular Degeneration | Details | |
| Bevacizumab biosimilar (Samsung Bioepis) | SP-8; SB-8 | Approved | Samsung Bioepis Co Ltd | Aybintio | EU | Fallopian Tube Neoplasms; Carcinoma, Ovarian Epithelial; Carcinoma, Non-Small-Cell Lung; Peritoneal Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Renal Cell | Samsung Bioepis Nl Bv | 2020-08-19 | Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Small Cell Lung Carcinoma; Breast Neoplasms; Peritoneal Neoplasms; Colorectal Neoplasms; Lung Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Ranibizumab biosimilar (Samsung Bioepis) | AM002; SB-11; SB11 | Approved | Samsung Bioepis Co Ltd | BYOOVIZ | EU | Macular Edema; Wet Macular Degeneration; Retinal Vein Occlusion; Choroidal Neovascularization; Diabetic macular oedema; Diabetic Retinopathy | Samsung Bioepis Nl Bv | 2021-08-18 | Macular Edema; Diabetic macular oedema; Wet Macular Degeneration; Macular Degeneration; Diabetic Retinopathy; Choroidal Neovascularization; Retinal Vein Occlusion | Details |
| Bevacizumab biosimilar (Intas Pharmaceuticals) | INTP-24 | Approved | Intas Biopharmaceuticals | Bevatas | India | Carcinoma, Non-Small-Cell Lung; Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms; Uterine Cervical Neoplasms; Glioblastoma; Carcinoma, Renal Cell; Breast Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms | Intas Biopharmaceuticals | 2017-10-04 | Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Glioblastoma; Breast Neoplasms; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Ranibizumab biosimilar (Formycon/Bioeq) | FYB-201 | Approved | Bioeq Gmbh | CIMERLI, Ranivisio | United States | Wet Macular Degeneration; Diabetic macular oedema; Macular Degeneration; Macular Edema; Retinal Vein Occlusion; Choroidal Neovascularization; Diabetic Retinopathy | Coherus Biosciences Inc | 2022-08-02 | Macular Edema; Wet Macular Degeneration; Diabetic macular oedema; Diabetes Complications; Macular Degeneration; Retinal Vein Occlusion; Choroidal Neovascularization; Diabetic Retinopathy | Details |
| Bevacizumab biosimilar (AryoGen Biopharma) | BE1040V | Approved | Aryogen Biopharma | Stivant | Iran | Carcinoma, Non-Small-Cell Lung; Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Colorectal Neoplasms; Uterine Cervical Neoplasms; Metastatic breast cancer; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Glioblastoma | Aryogen Biopharma | 2019-06-01 | Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Glioblastoma; Colorectal Neoplasms; Peritoneal Neoplasms; Metastatic breast cancer; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Bevacizumab biosimilar (Zydus Cadila) | Approved | Zydus Cadila | Bryxta | India | Carcinoma, Non-Small-Cell Lung; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms; Colorectal Neoplasms; Glioblastoma; Metastatic breast cancer; Carcinoma, Renal Cell | Zydus Cadila | 2017-01-01 | Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Glioblastoma; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Metastatic breast cancer; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details | |
| Bevacizumab biosimilar (TOT Biopharm) | TAB-008; TOT-102; TAB008; TOT102 | Approved | 朴欣汀, Pusintin | Mainland China | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Tot Biopharm Co Ltd | 2021-12-01 | Carcinoma, Ovarian Epithelial; Glioblastoma; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details | |
| Bevacizumab | G180CU; RO-4876646; RG-435; NSC-704865; G180CL; R-435; G180DL | Approved | Genentech Inc | 安维汀, Avastin | United States | Colorectal Neoplasms | Genentech Inc | 2004-02-26 | Solid tumours; Liver Neoplasms; Ovarian Neoplasms; Recurrence; Infections; Respiratory Tract Infections; Pterygium; Leukemia; Arteriovenous Malformations; Ependymoma; Telangiectasia, Hereditary Hemorrhagic; HIV Infections; Leukemia, Myelogenous, Chronic; Kidney Neoplasms; Epistaxis; Head and Neck Neoplasms; Leiomyosarcoma; Fibrosarcoma; Telangiectasis; Meningeal Carcinomatosis; Rectal Neoplasms; Vaginal Neoplasms; Esophageal Neoplasms; Macular Edema; Anaplasia; Carcinoma, Renal Cell; Diabetes Mellitus, Type 2; Squamous Cell Carcinoma of Head and Neck; Abdominal Neoplasms; Granuloma, Lethal Midline; Hemangioblastoma; Carcinoma; Carcinoma, Basal Cell; Esthesioneuroblastoma, Olfactory; Carcinoid Tumor; Carcinoma, Ovarian Epithelial; Glioblastoma; Neurofibromatosis 2; Pancreatic Neoplasms; Neoplasms, Glandular and Epithelial; Virus Diseases; Papillomavirus Infections; Neoplasms, Squamous Cell; Respiratory Tract Diseases; Neoplasms; Small Cell Lung Carcinoma; Myelodysplastic Syndromes; Triple Negative Breast Neopl | Details |
| Bevacizumab biosimilar(Dr. Reddy's Laboratories) | DRL-BZ | Approved | Dr.Reddy's Laboratories Ltd | Versavo, Persivia | India | Fallopian Tube Neoplasms; Uterine Cervical Neoplasms; Glioblastoma; Peritoneal Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Ovarian Epithelial; Carcinoma, Non-Small-Cell Lung; Carcinoma, Renal Cell | Dr.Reddy's Laboratories Ltd | 2019-08-19 | Ovarian Neoplasms; Carcinoma, Renal Cell; Glioblastoma; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Bevacizumab biosimilar (Celltrion) | CTP-16; CT-16; CT-P16 | Approved | Celltrion Inc | Vegzelma | EU | Carcinoma, Renal Cell; Peritoneal Neoplasms; Ovarian Neoplasms; Carcinoma, Non-Small-Cell Lung; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms; Breast Neoplasms; Colorectal Neoplasms | Celltrion Healthcare Hungary Kft | 2022-08-17 | Ovarian Neoplasms; Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Glioblastoma; Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Aflibercept biosimilar (Momenta/Mylan) | M-710; MYL-1701P | Approved | Momenta, Mylan Nv | EU | Diabetes Complications; Myopia, Degenerative; Diabetic Retinopathy; Macular Edema; Retinal Vein Occlusion | Viatris Ltd | 2023-09-15 | Macular Edema; Myopia, Degenerative; Diabetic macular oedema; Diabetes Complications; Diabetic Retinopathy; Retinal Vein Occlusion | Details | |
| Ranibizumab biosimilar (Intas Biopharmaceuticals) | Approved | Intas Biopharmaceuticals | Razumab | India | Macular Degeneration | Intas Biopharmaceuticals | 2015-01-01 | Macular Degeneration | Details | |
| Ranibizumab biosimilar (Xbrane) | Approved | Xbrane Biopharma Ab | EU | Diabetic Retinopathy; Macular Edema; Wet Macular Degeneration; Diabetes Complications | Stada Arzneimittel Ag | 2022-11-09 | Macular Edema; Wet Macular Degeneration; Diabetes Complications; Macular Degeneration; Diabetic Retinopathy | Details | ||
| Bevacizumab biosimilar (Boan Biopharma) | LY-01008 | Approved | 博优诺, Boyounuo | Mainland China | Carcinoma, Non-Small-Cell Lung; Colorectal Neoplasms | Shandong Boan Biotechnology Co Ltd | 2021-05-07 | Carcinoma, Ovarian Epithelial; Glioblastoma; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Carcinoma, Hepatocellular | Details | |
| Bevacizumab biosimilar (Shanghai Henlius Biotech) | HLX-04; HLX04-O | Approved | Shanghai Henlius Biotech Inc | 汉贝泰 | Mainland China | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Shanghai Henlius Biopharmaceuticals Co Ltd | 2021-11-30 | Solid tumours; Carcinoma; Rectal Neoplasms; Carcinoma, Ovarian Epithelial; Glioblastoma; Wet Macular Degeneration; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Macular Degeneration; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| Bevacizumab biosimilar (Biocad) | BCD-021 | Approved | Biocad | Avegra | Russian Federation | Glioblastoma; Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Fallopian Tube Neoplasms; Peritoneal Neoplasms; Uterine Cervical Neoplasms | Biocad | 2015-11-25 | Carcinoma, Renal Cell; Glioblastoma; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Macular Degeneration; Uterine Cervical Neoplasms | Details |
| Bevacizumab biosimilar (Sinocelltech) | SCT-510; SCT510A; SCT-510A | Approved | SinoCelltech Ltd | 安贝珠 | Mainland China | Peritoneal Neoplasms; Uterine Cervical Neoplasms; Ovarian Neoplasms; Fallopian Tube Neoplasms; Glioblastoma; Colorectal Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | SinoCelltech Ltd | 2023-06-27 | Ovarian Neoplasms; Glioblastoma; Carcinoma, Ovarian Epithelial; Wet Macular Degeneration; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Macular Degeneration; Uterine Cervical Neoplasms; Carcinoma, Hepatocellular | Details |
| Ranibizumab biosimilar (Qilu Pharmaceutical) | BCD-300; QL-1205 | Approved | Biocnd, Qilu Pharmaceutical Co Ltd | Rimmyrah | EU | Myopia, Degenerative; Choroidal Neovascularization; Diabetes Complications; Macular Edema; Wet Macular Degeneration | Qilu Pharma Spain Sa | 2024-01-05 | Macular Edema; Myopia, Degenerative; Wet Macular Degeneration; Diabetic macular oedema; Diabetes Complications; Retinal Vein Occlusion; Macular Degeneration; Choroidal Neovascularization; Diabetic Retinopathy; Myopia | Details |
| Bevacizumab biosimilar (Allergan/Amgen) | ABP-215 | Approved | Amgen Inc | Mvasi | United States | Glioblastoma; Carcinoma, Renal Cell; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Amgen Inc | 2017-09-14 | Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Glioblastoma; Breast Neoplasms; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Aflibercept biosimilar (Qilu Pharmaceutical) | QL-1207 | Approved | Qilu Pharmaceutical Co Ltd | Mainland China | Macular Degeneration; Diabetic macular oedema | Qilu Pharmaceutical Co Ltd | 2023-12-13 | Diabetic macular oedema; Macular Degeneration | Details | |
| Ranibizumab biosimilar (Senju) | GBS-007; OT-701; SJP-0133 | Approved | Senju Pharmaceutical Co Ltd | Japan | Macular Degeneration | Senju Pharmaceutical Co Ltd | 2021-09-27 | Wet Macular Degeneration; Macular Degeneration | Details | |
| Bevacizumab biosimilar (mAbixience) | MB02; BEVZ-92; BEVZ92-MB02; AP-01 | Approved | Mabxience Sa | Alymsys | Argentina | Colorectal Neoplasms; Carcinoma, Renal Cell; Peritoneal Neoplasms; Carcinoma, Non-Small-Cell Lung; Breast Neoplasms; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms | Stada Arzneimittel Ag | 2013-10-25 | Carcinoma, Renal Cell; Glioblastoma; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Neovasculgen | PI-VEGF165 | Approved | Human Stem Cells Institute | Neovasculgen | Russian Federation | Peripheral Arterial Disease | Human Stem Cells Institute | 2011-12-07 | Peripheral Arterial Disease; Peripheral Nerve Injuries | Details |
| Bevacizumab biosimilar (CTTQ Pharma) | TQ-B2302 | Approved | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 安倍斯 | Mainland China | Glioblastoma; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Nanjing Shunxin Pharmaceuticals Co Ltd Of Chiatai Tianqing Pharmaceutical Group | 2023-02-28 | Ovarian Neoplasms; Glioblastoma; Neoplasms; Colorectal Neoplasms; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Bevacizumab biosimilar (Biocon/Mylan) | MYL-1402O | Approved | Biocon Ltd | Lextemy, KRABEVA, Abevmy | India | Carcinoma, Renal Cell; Colorectal Neoplasms; Ovarian Neoplasms; Brain Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Mylan Pharmaceuticals Private Ltd | 2017-11-27 | Ovarian Neoplasms; Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Brain Neoplasms; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Conbercept | FP-3; KH-902 | Approved | Chengdu Kanghong Biotechnologies Co Ltd | 朗沐, Langmu | Mainland China | Macular Degeneration | Chengdu Kanghong Biotechnologies Co Ltd | 2013-11-27 | Macular Edema; Vision Disorders; Wet Macular Degeneration; Diabetic macular oedema; Retinoblastoma; Hemangioma; Uveitis; Retinal Vein Occlusion; Choroidal Neovascularization; Corneal Neovascularization; Diabetes Mellitus; Macular Degeneration | Details |
| Bevacizumab biosimilar(Apotex ) | Approved | Apotex Inc | BAMBEVI | Canada | Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms; Glioblastoma; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Apotex Inc | 2021-09-23 | Carcinoma, Ovarian Epithelial; Glioblastoma; Peritoneal Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| Aflibercept | BAY-865321; BAT-86-5321 | Approved | Bayer AG | Eylea, 艾力雅 | United States | Macular Degeneration; Macular Edema | Regeneron Pharmaceuticals Inc | 2011-11-18 | Retinitis Pigmentosa; Choroidal Neovascularization; Carcinoma, Non-Small-Cell Lung; Corneal Neovascularization; Retinopathy of Prematurity; Neoplasm Metastasis; Diabetic Retinopathy; Retinal Vein Occlusion; Macular Degeneration; Vitreous Hemorrhage; Lymphoma, Non-Hodgkin; Retinal Degeneration; Eye Diseases; Diabetes Complications; Colorectal Neoplasms; Prostatic Neoplasms; Diabetes Mellitus, Type 1; Wet Macular Degeneration; Diabetic macular oedema; Multiple Myeloma; Myopia, Degenerative; Retinal Diseases; Colonic Neoplasms; Central Serous Chorioretinopathy; Neoplasms; Diabetes Mellitus, Type 2; Rectal Neoplasms; Choroid Diseases; Glaucoma, Neovascular; Macular Edema; Cataract | Details |
| Bevacizumab biosimilar (Jiangsu Hengrui Medicine) | BP-102 | Approved | Jiangsu Hengrui Medicine Co Ltd | 艾瑞妥 | Mainland China | Carcinoma, Non-Small-Cell Lung; Colorectal Neoplasms | Suzhou Suncadia Biopharmaceuticals Co Ltd | 2021-06-22 | Solid tumours; Triple Negative Breast Neoplasms; Colorectal Neoplasms; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Ziv-aflibercept | BAY-865321; AVE-0005 | Approved | Sanofi | Zaltrap | United States | Colorectal Neoplasms | Sanofi-Aventis U.S. Llc | 2012-08-03 | Lymphoma; Breast Neoplasms; Urethral Neoplasms; Brain Neoplasms; Colorectal Neoplasms; Genital Neoplasms, Female; Carcinoma, Mucoepidermoid; Gliosarcoma; Astrocytoma; Leukemia, Myeloid, Chronic, Atypical, BCR-ABL Negative; Peritoneal Neoplasms; Ureteral Neoplasms; Lung Neoplasms; Prostatic Neoplasms; Uterine Neoplasms; Endometrial Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Neuroendocrine; Glioma; Lymphoma, Non-Hodgkin; Thyroid Neoplasms; Carcinoma, Squamous Cell; Retinal Vein Occlusion; Neoplasm Metastasis; Melanoma; Carcinoma, Non-Small-Cell Lung; Glioblastoma; Solid tumours; Leiomyosarcoma; Multiple Endocrine Neoplasia Type 1; Leukemia; Carcinoma; Carcinoma, Renal Cell; Carcinoid Tumor; Rectal Neoplasms; Neoplasms; Carcinoma, Ovarian Epithelial; Pancreatic Neoplasms; Leukemia, Myelomonocytic, Chronic; Ovarian Neoplasms; Myelodysplastic Syndromes; Myeloproliferative Disorders; Carcinoma, Transitional Cell; Carcinoma, Papillary; Small Cell Lung Carcinoma; Colonic Neoplasms; Ascites; Lung Diseases; Adenoc | Details |
| Ranibizumab | RG-3645; rhu-Fab-VEG; AMD-rhuFab-V2; AMD-Fab; rhuFab-V2; RFB-002; Y-0317; RG-6321 | Approved | Novartis Pharma Ag, Genentech Inc | Lucentis, 诺适得, Susvimo | United States | Macular Degeneration | Genentech Inc | 2006-06-30 | Retinal Degeneration; Wet Macular Degeneration; Retinoblastoma; Vascular Diseases; Glaucoma; Retinal Detachment; Diabetes Complications; Pseudoxanthoma Elasticum; Retinal Neovascularization; Cardiovascular Diseases; Eye Diseases; Uveitis; Ischemia; Vitreous Hemorrhage; Hemangioma; Retinopathy of Prematurity; Diabetic Retinopathy; Choroidal Neovascularization; Retinal Vein Occlusion; Macular Degeneration; Melanoma; Conjunctival Neoplasms; Port-Wine Stain; Corneal Neovascularization; von Hippel-Lindau Disease; Myopia; Diabetes Mellitus; Telangiectasis; Cataract; Pterygium; Polypoidal choroidal vasculopathy; Diabetic Angiopathies; Retinal Telangiectasis; Vitreous Detachment; Epistaxis; Telangiectasia, Hereditary Hemorrhagic; Iris Diseases; Vision Disorders; Macular Edema; Glaucoma, Neovascular; Optic Neuropathy, Ischemic; Strongyloidiasis; Neovascularization, Pathologic; Depression; Central Serous Chorioretinopathy; Histoplasmosis; Retinal Diseases; Angioid Streaks; Myopia, Degenerative; Pathologic Processes; Mu | Details |
| Bevacizumab biosimilar (Reliance Life Sciences) | R-TPR-023 | Approved | Reliance Life Sciences | BevaciRel | India | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Glioblastoma; Carcinoma, Renal Cell; Fallopian Tube Neoplasms; Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms; Uterine Cervical Neoplasms | Reliance Life Sciences Pvt Ltd | 2016-06-13 | Carcinoma, Renal Cell; Glioblastoma; Carcinoma, Ovarian Epithelial; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Faricimab | RO-6867461; RG-7716 | Approved | F. Hoffmann-La Roche Ltd, Genentech Inc | Vabysmo, 罗视佳 | United States | Diabetic macular oedema; Wet Macular Degeneration | Genentech Inc | 2022-01-28 | Polypoidal choroidal vasculopathy; Macular Edema; Choroid Diseases; Vision Disorders; Retinal Diseases; Diabetic macular oedema; Wet Macular Degeneration; Diabetes Complications; Macular Degeneration; Diabetes Mellitus; Retinal Vein Occlusion; Diabetic Retinopathy; Choroidal Neovascularization | Details |
| Bevacizumab biosimilar (Hetero Drugs) | Approved | Hetero Drugs Ltd | Cizumab | India | Colorectal Neoplasms | Hetero Drugs Ltd | 2016-06-27 | Colorectal Neoplasms | Details | |
| Bevacizumab biosimilar (Bio-Thera Solutions) | BAT-1706 | Approved | Bio-Thera Solutions Ltd | 普贝希, AVZIVI | Mainland China | Carcinoma, Non-Small-Cell Lung; Colorectal Neoplasms | Bio-Thera Solutions Ltd, Beigene Ltd | 2021-11-17 | Ovarian Neoplasms; Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Glioblastoma; Neoplasms; Breast Neoplasms; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Carcinoma, Hepatocellular | Details |
| Bevacizumab biosimilar (Betta/Mabworks) | MIL-60 | Approved | Institute Of Basic Medicine, Chinese Academy Of Medical Sciences, Beijing Mabworks Biotech Co Ltd | 贝安汀 | Mainland China | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Betta Pharmaceuticals Co Ltd | 2021-11-24 | Carcinoma, Ovarian Epithelial; Glioblastoma; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Bevacizumab biosimilar(Jiangsu Aosaikang) | ASK-1202; AMD-B; AK-3008; ASK-B1202; ASKB1202 | Phase 3 Clinical | Jiangsu Aosaikang Pharmaceutical Co Ltd | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Ranibizumab biosimilar (Reliance Life Sciences Group) | R-TPR-024 | Phase 3 Clinical | Reliance Life Sciences | Macular Degeneration | Details |
| TR-009 | NOV-1501; ABL001-ABL Bio; ABL-001-ABL Bio; ES-104; CTX-009; HD-B001A; HDB001A; TR-009 | Phase 3 Clinical | Abl Bio Inc | Biliary Tract Neoplasms; Solid tumours; Rectal Neoplasms; Colonic Neoplasms; Neoplasms; Cholangiocarcinoma; Bile Duct Neoplasms; Colorectal Neoplasms; Gallbladder Neoplasms | Details |
| Bevacizumab biosimilar (Fudan-Zhangjiang) | Phase 3 Clinical | Shanghai Fudan-Zhangjiang Bio-Pharmaceutical Co Ltd | Colorectal Neoplasms; Macular Degeneration; Carcinoma, Non-Small-Cell Lung | Details | |
| Aflibercept Biosimilar (Alteogen) | ALT-L9 | Phase 3 Clinical | Alteogen Inc, Kissei Pharmaceutical Co Ltd | Macular Degeneration | Details |
| Bevacizumab biosimilar (Huaota Biopharm/Shanghai Junshi Biosciences) | JS-501; HOT-1010 | Phase 3 Clinical | Shanghai Huaota Biopharmaceutical Co Ltd | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Bevacizumab biosimilar (Prestige BioPharma/Hanwha Biologics) | HD-204 | Phase 3 Clinical | Hanwha Biologics | Small Cell Lung Carcinoma; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Bevacizumab biosimilar (Oncobiologics) | ONS-5010; ONS-1045 | Phase 3 Clinical | Outlook Therapeutics Inc | Diabetic macular oedema; Wet Macular Degeneration; Retinal Vein Occlusion; Macular Degeneration | Details |
| Aflibercept biosimilar (CinnaGen) | Phase 3 Clinical | Cinnagen | Macular Degeneration | Details | |
| Bevacizumab biosimilar (Alphamab/R-Pharm) | RPH-001 | Phase 3 Clinical | Suzhou Alphamab Co Ltd, R-Pharm | Colorectal Neoplasms | Details |
| Bevacizumab biosimilar(Zhejiang Teruisi Pharmaceutical) | TRS-003 | Phase 3 Clinical | Zhejiang Teruisi Pharmaceutical Inc | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Ranibizumab biosimilar (Chong Kun Dang Pharmaceutical) | CKD-701 | Phase 3 Clinical | Chong Kun Dang Pharmaceutical Corp | Macular Degeneration | Details |
| Ranibizumab biosimilar (Lupin) | LUBT-010 | Phase 3 Clinical | Lupin Ltd | Macular Degeneration | Details |
| Abicipar pegol | MP-0112; AGN-150998 | Phase 3 Clinical | Molecular Partners Ag | Macular Edema; Diabetic macular oedema; Macular Degeneration | Details |
| Bevacizumab biosimilar (Genor Biopharma) | GB-222 | Phase 3 Clinical | Genor Biopharma Co Ltd | Brain Neoplasms; Glioma; Carcinoma, Non-Small-Cell Lung | Details |
| Muparfostat sodium | PI-88 | Phase 3 Clinical | Australian National University | Solid tumours; Liver Neoplasms; Neoplasms; Small Cell Lung Carcinoma; Prostatic Neoplasms; Lung Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Melanoma | Details |
| Aflibercept biosimilar (Amgen) | ABP-938 | Phase 3 Clinical | Amgen Inc | Vascular Diseases; Macular Degeneration | Details |
| Bevacizumab biosimilar (Centus Biotherapeutics) | FKB-238 | Phase 3 Clinical | Fujifilm Kyowa Kirin Biologics Co Ltd | Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Bevacizumab biosimilar(Laboratorios Sophia) | PRO-169 | Phase 3 Clinical | Laboratorios Sophia Sa De Cv | Diabetic macular oedema | Details |
| Aflibercept Biosimilar(Alvotech Swiss) | AVT-06 | Phase 3 Clinical | Alvotech Swiss Ag | Vascular Diseases; Macular Degeneration | Details |
| Aflibercept biosimilar (HEXAL/Sandoz) | SOK-583A1; SOK583A1; SOK-583 | Phase 3 Clinical | Sandoz, Hexal | Macular Degeneration | Details |
| CT-P42 | CT-P42 | Phase 3 Clinical | Celltrion Inc | Macular Edema; Diabetic macular oedema | Details |
| Aflibercept biosimilar (Mabwell) | 9-MW-0813; 9MW-0813; 9MW0813; 9-MW0813 | Phase 3 Clinical | Mabwell (Shanghai) Bioscience Co Ltd | Diabetic macular oedema | Details |
| Bevacizumab biosimilar (Beijing Science Sun/Beijing Lvzhu) | K-11 | Phase 3 Clinical | Beijing Lvzhu Biological Technology Co Ltd, Beijing Science Sun Pharmaceutical Co Ltd | Liver Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| FYB-203 | FYB-203 | Phase 3 Clinical | Macular Degeneration | Details | |
| Aflibercept biosimilar (Sam Chun Dang Pharm) | SCD-411 | Phase 3 Clinical | Sam Chun Dang Pharm Co Ltd | Wet Macular Degeneration; Macular Degeneration | Details |
| Aflibercept biosimilar (Samsung Bioepis) | SB-15; AM003; SB15 | Phase 3 Clinical | Samsung Bioepis Co Ltd | Solid tumours; Wet Macular Degeneration; Diabetic macular oedema; Macular Degeneration | Details |
| Aflibercept Biosimilar (Boan Biopharma/Luye Pharma) | LY-09004; BA-9101; OT-702 | Phase 3 Clinical | Luye Pharma Group Ltd | Wet Macular Degeneration; Macular Degeneration | Details |
| Varisacumab | R-84; GNR-011; AT-001-IBCG | Phase 3 Clinical | Peregrine, The University Of Texas Southwestern Medical Center | Glioblastoma; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| MP-0250 | MP-0250 | Phase 2 Clinical | Molecular Partners Ag | Neoplasms; Multiple Myeloma; Carcinoma, Non-Small-Cell Lung | Details |
| ALS-L1023 | ALS-L1023 | Phase 2 Clinical | Angiolab Inc | Sleep Bruxism; Metabolic Dysfunction-Associated Steatotic Liver Disease; Metabolic Syndrome; Temporomandibular Joint Disorders; Macular Degeneration | Details |
| Bevacizumab biosimilar (Eastern Biotech) | JY-028 | Phase 2 Clinical | Beijing Eastern Biotech Co Ltd | Wet Macular Degeneration; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Macular Degeneration | Details |
| Intravitreal ranibizumab (Sultan Qaboos University) | Phase 2 Clinical | Sultan Qaboos University | Retinal Dystrophies | Details | |
| ELGN-EYE | ELGN-EYE | Phase 2 Clinical | Elgan Pharma Ltd | Retinopathy of Prematurity | Details |
| SCT-501(National Cancer Institute) | SCT-501 | Phase 2 Clinical | National Cancer Institute | Kidney Neoplasms | Details |
| Y-400 | Y-400 | Phase 2 Clinical | Wuhan Yzy Biopharma Co Ltd | Wet Macular Degeneration; Diabetic macular oedema; Macular Degeneration | Details |
| BI-836880 | BI-836880 | Phase 2 Clinical | Ablynx Nv | Neoplasms; Wet Macular Degeneration | Details |
| SYB-509 | SYB-509 | Phase 1 Clinical | Sichuan Yuanda Shuyang Pharmaceutical Co Ltd | Carcinoma, Transitional Cell; Carcinoma, Hepatocellular | Details |
| Vanucizumab | RG-7221; RO-5520985; B800Z06O8K (UNII code) | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Solid tumours; Neoplasms; Colorectal Neoplasms | Details |
| Bevacizumab biosimilar (North China Pharmaceutical) | MG-021 | Phase 1 Clinical | North China Pharmaceutical Company Ltd | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Macular Degeneration | Details |
| Bevacizumab biosimilar(Guilin Sanjin) | Phase 1 Clinical | Guilin Sanjin Pharmaceutical Co Ltd | Macular Degeneration | Details | |
| Ranibizumab biosimilar (CJSC Generium) | GNR-067 | Phase 1 Clinical | Cjsc Generium | Macular Degeneration | Details |
| Bevacizumab biosimilar (Shanghai Kangdai) | Phase 1 Clinical | Shanghai Kanda Bio-Technology Co Ltd | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| Bevacizumab biosimilar (JHL Biotech) | JHL-1149 | Phase 1 Clinical | JHL Biotech | Neoplasms | Details |
| Ziv-aflibercept biosimiliar (Boan Biopharma) | LY-01012; BA-1103 | Phase 1 Clinical | Colorectal Neoplasms | Details | |
| Bevacizumab biosimilar (Tanvex BioPharma) | TX-16 | Phase 1 Clinical | Tanvex Biopharma | Colorectal Neoplasms | Details |
| PB-101 | PB-101 | Phase 1 Clinical | Panolos Bioscience Inc | Solid tumours; Stomach Neoplasms; Neoplasms; Colorectal Neoplasms; Carcinoma, Hepatocellular | Details |
| IBI-333 | IBI-333 | Phase 1 Clinical | Innovent Biologics (Usa), Inc | Wet Macular Degeneration; Macular Degeneration | Details |
| IBI-324 | IBI-324 | Phase 1 Clinical | Innovent Biologics (Usa), Inc | Diabetic macular oedema | Details |
| Y-332 | Y-332 | Phase 1 Clinical | CSPC Pharmaceutical Group Ltd, Wuhan Yzy Medical Science And Technology Co Ltd | Solid tumours | Details |
| Dilpacimab | ABT-165; DVD-Ig ABT-165 | Phase 1 Clinical | Abbvie Inc | Solid tumours; Neoplasms | Details |
| Bevacizumab biosimilar (Gedeon Richter) | Phase 1 Clinical | Gedeon Richter Plc | Neoplasms | Details | |
| HG-202 | HG-202; HG202 | Phase 1 Clinical | HuiGene Therapeutics Ltd | Wet Macular Degeneration; Macular Degeneration | Details |
| Aflibercept biosimilar (Zein Bioteccnology) | Phase 1 Clinical | Zein Bioteccnology Co Ltd | Diabetic macular oedema | Details | |
| VEGFA-targeting Gene Therapy(BDgene) | BD311 | Phase 1 Clinical | Shanghai BDgene Technology Co Ltd | Diabetic macular oedema; Macular Degeneration; Retinal Vein Occlusion | Details |
| ASKG-712 | ASKG-712; AM-712 | Phase 1 Clinical | Askgene Pharma | Diabetic macular oedema; Wet Macular Degeneration; Macular Degeneration | Details |
| OB-318 | OB-318 | Phase 1 Clinical | Oneness Biotech Co Ltd | Solid tumours; Carcinoma, Hepatocellular | Details |
| Bevacizumab biosimilar(Guangdong Dongyangguang) | Phase 1 Clinical | Guangdong Dongyangguang Pharmaceutical Co Ltd | Carcinoma, Non-Small-Cell Lung | Details | |
| Colorectal cancer vaccine (Immunovo/Pepscan Therapeutics) | Phase 1 Clinical | Pepscan Systems | Colorectal Neoplasms | Details | |
| Bevacizumab biosimilar(Bioxpress) | BXT-2316 | Clinical | Bioxpress Therapeutics Sa | Carcinoma, Renal Cell; Glioblastoma; Carcinoma, Ovarian Epithelial; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
This web search service is supported by Google Inc.
