 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
| GMP-L12H23 | Human | GMP Human IL-12 Protein |
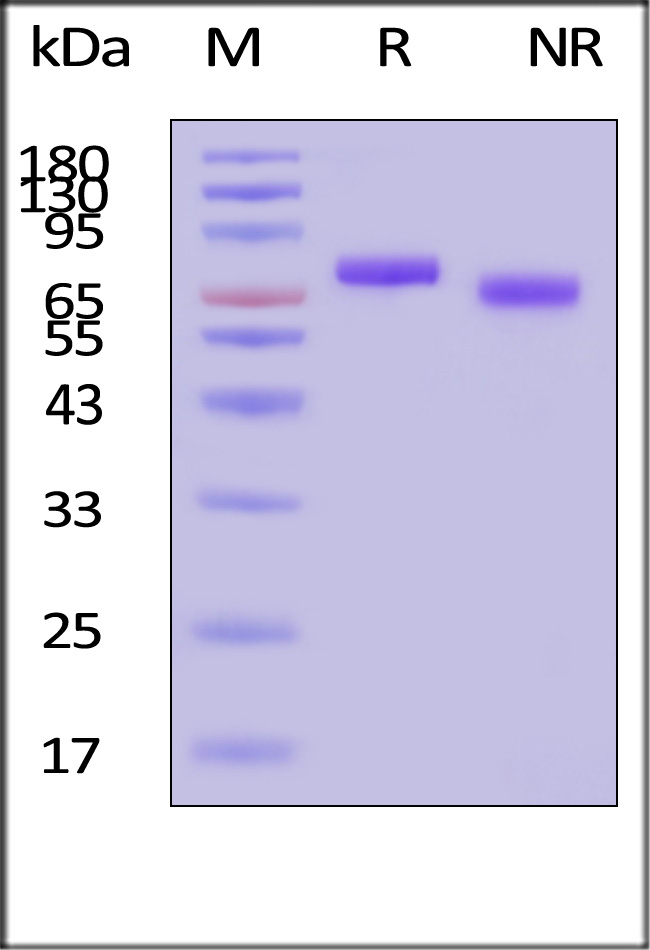
|
||
| IL2-H5210 | Human | Human IL-12 Protein, premium grade |
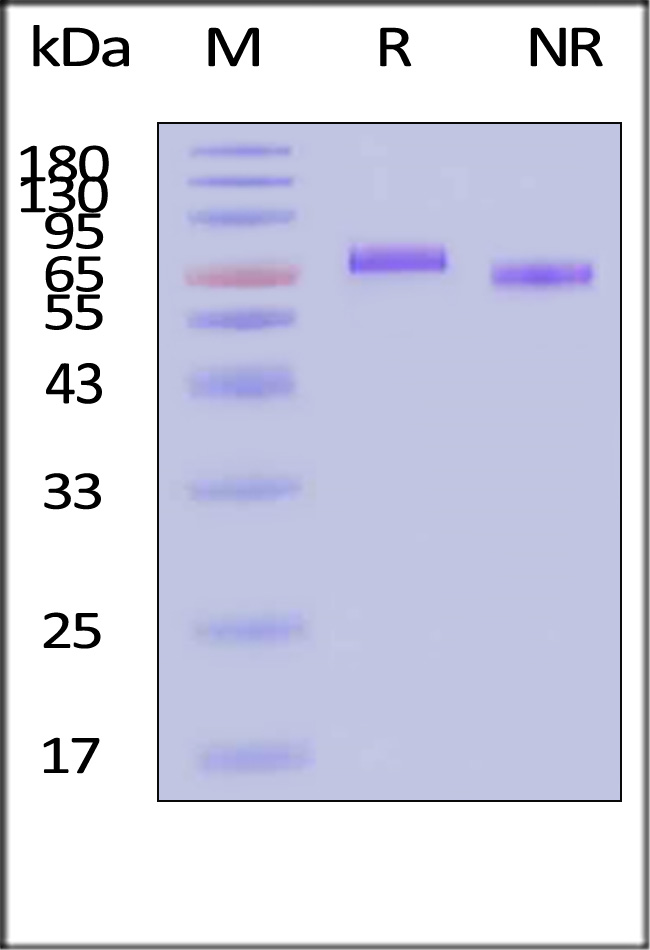
|
||
| IL2-H8210 | Human | Biotinylated Human IL-12B&IL-12A Heterodimer Protein, His,Avitag™&Flag Tag |  |
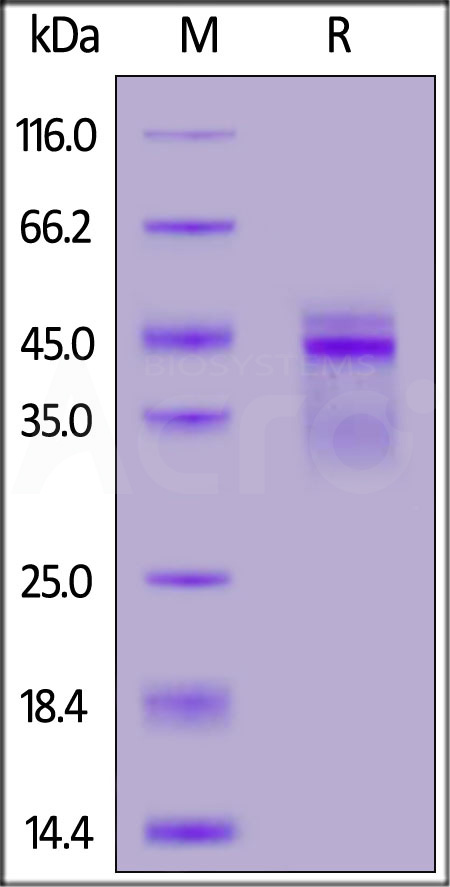
|
|
| IL2-H4210 | Human | Human IL-12B&IL-12A Heterodimer Protein, His Tag&Flag Tag (MALS verified) |  |
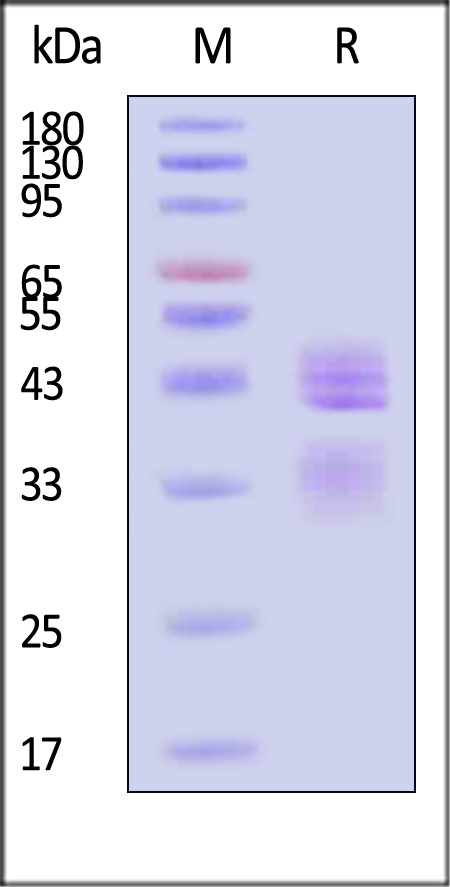
|
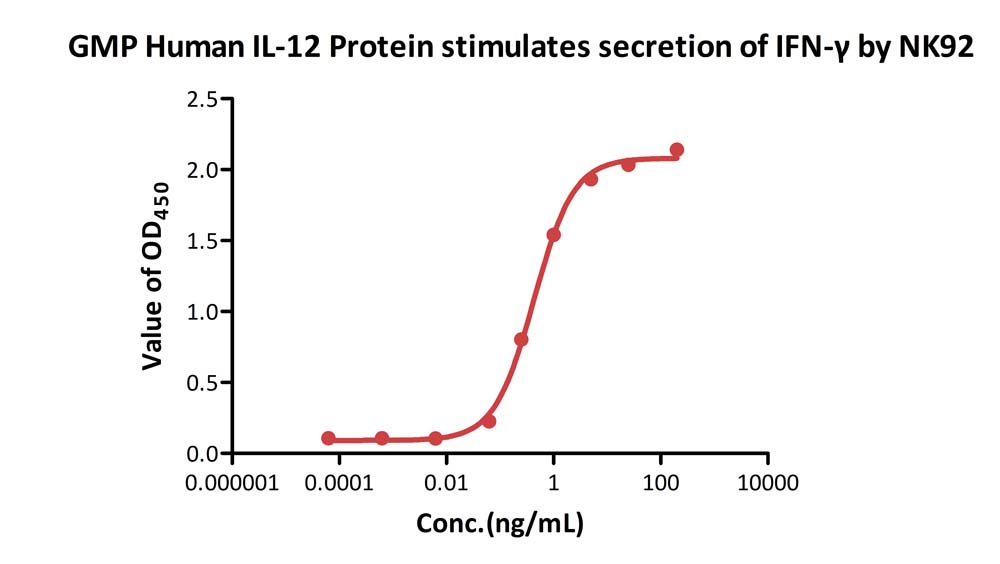
GMP Human IL-12 Protein (Cat. No. GMP-L12H23) stimulates secretion of IFN-γ by NK-92. The specific activity of GMP Human IL-12 Protein is > 1.00ⅹ10^7 IU/mg, which is calibrated against human IL-12 WHO International Standard (NIBSC code: 95/544) (QC tested).
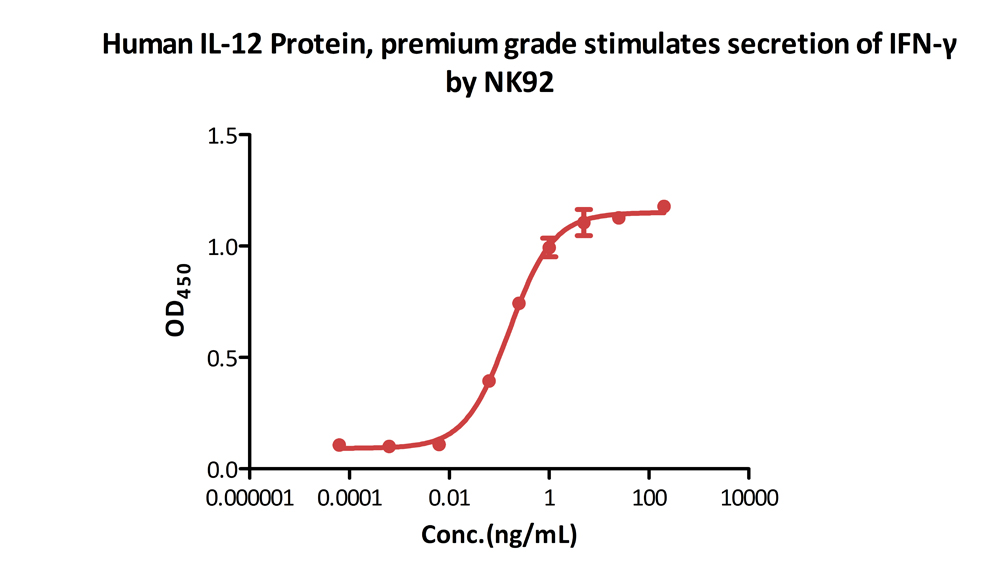
Human IL-12 Protein, premium grade (Cat. No. IL2-H5210) stimulates secretion of IFN-γ by NK-92 human natural killer lymphoma cells. The specific activity of Human IL-12 Protein, premium grade is > 1.00ⅹ10^7 IU/mg, which is calibrated against human IL-12 WHO International Standard (NIBSC code: 95/544) (QC tested).
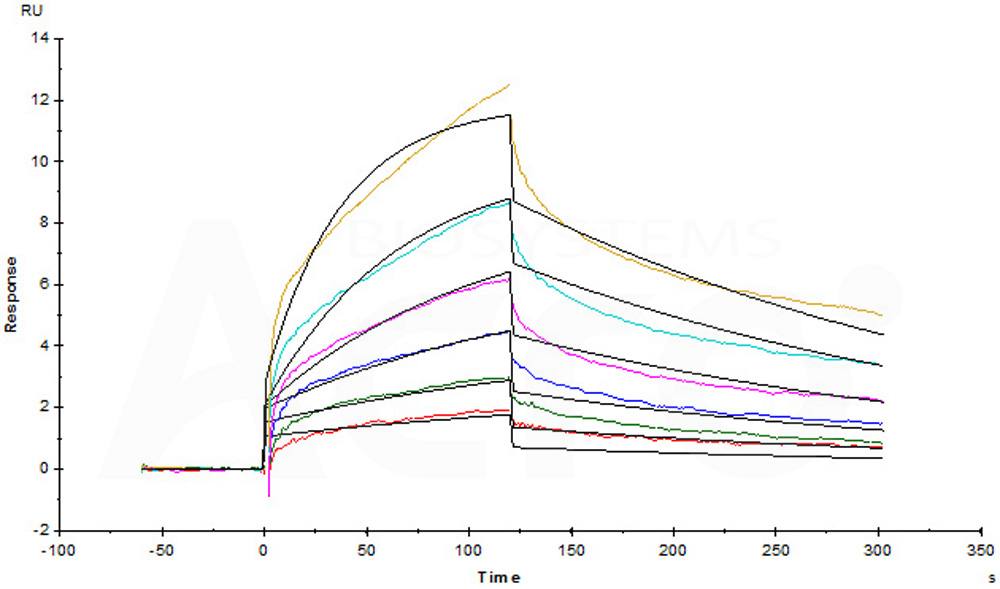
Human IL-12 R beta 1, Fc Tag (Cat. No. ILB-H5255) captured on CM5 chip via Anti-human IgG Fc antibodies surface can bind Human IL-12B&IL-12A Heterodimer Protein, His Tag&Flag Tag (Cat. No. IL2-H4210) with an affinity constant of 2.06 nM as determined in a SPR assay (Biacore T200) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Ustekinumab | CNTO-1275; C-340 | Approved | Johnson & Johnson Innovative Medicine | Stelara, 喜达诺 | United States | Plaque psoriasis | Janssen Biotech Inc | 2009-09-25 | Arthritis, Juvenile; Crohn Disease; Dermatitis, Atopic; Plaque psoriasis; Hidradenitis Suppurativa; Uveitis; Common Variable Immunodeficiency; Colitis, Ulcerative; Arthritis, Psoriatic; Takayasu Arteritis; Psoriasis; Lupus Erythematosus, Systemic; Liver Cirrhosis, Biliary; Multiple Sclerosis; Sjogren's Syndrome; Pouchitis; Ichthyosis; Inflammatory Bowel Diseases; Type I Leukocyte Adhesion Defect; Dermatomyositis; Non-radiographic axial spondyloarthritis; Diabetes Mellitus, Type 1; Polymyositis | Details |
| Ustekinumab biosimilar (Alvotech) | AVT04; AVT-04 | Approved | Alvotech hf | Jamteki, Uzpruvo, SELARSDI™ | Japan | Psoriasis | Fuji Pharma Co Ltd | 2023-09-25 | Psoriasis; Arthritis, Psoriatic; Plaque psoriasis; Crohn Disease | Details |
| Ustekinumab biosimilar(Amgen) | ABP-654 | Approved | Amgen Inc | Wezlana, WEZLANA | United States | Crohn Disease; Plaque psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative | Amgen Inc | 2023-10-31 | Psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative; Crohn Disease; Plaque psoriasis | Details |
| Thalidomide | NSC-66847; NSC-527179; K-17; VP-02; FPF-300; FPF300 | Approved | Celgene Corp | Talizer, Thalidomide Celgene, Thalidomide Pharmion, Synovir, Thalomid, Thaled | Mainland China | Leprosy, Lepromatous; Multiple Myeloma | Changzhou Pharmaceutical Factory | 1982-01-01 | HIV Wasting Syndrome; Angiodysplasia; Primary Myelofibrosis; Neuroectodermal Tumors, Primitive; Prostatitis; Colorectal Neoplasms; Osteosarcoma; Lymphoma, Mantle-Cell; Sarcoma, Ewing; Retinoblastoma; Erythema Nodosum; Drug Resistant Epilepsy; Xerostomia; Sarcoma; Pancreatitis, Chronic; Adenocarcinoma, Clear Cell; Lymphoma, Follicular; Arachnoiditis; Carcinoma, Adenosquamous; Gastrointestinal Hemorrhage; Cholangitis, Sclerosing; Prostatic Neoplasms; Pelvic Pain; Neoplasm Metastasis; Stomatitis; Burning Mouth Syndrome; Mycobacterium avium-intracellulare Infection; Amyotrophic Lateral Sclerosis; Melanoma; Myelodysplastic-Myeloproliferative Diseases; Carcinoma, Hepatocellular; Leukemia, Lymphocytic, Chronic, B-Cell; Vascular Malformations; Tuberculosis; Appendiceal Neoplasms; Lymphoma, Non-Hodgkin; Uterine Neoplasms; Anemia, Sideroblastic; Glioma; Leprosy, Lepromatous; Endometrial Neoplasms; Lung Neoplasms; Waldenstrom Macroglobulinemia; Kidney Neoplasms; Thalassemia; Carcinoid Tumor; Lupus Erythematosus, Discoid | Details |
| Ustekinumab | CNTO-1275; C-340 | Approved | Johnson & Johnson Innovative Medicine | Stelara, 喜达诺 | United States | Plaque psoriasis | Janssen Biotech Inc | 2009-09-25 | Arthritis, Juvenile; Crohn Disease; Dermatitis, Atopic; Plaque psoriasis; Hidradenitis Suppurativa; Uveitis; Common Variable Immunodeficiency; Colitis, Ulcerative; Arthritis, Psoriatic; Takayasu Arteritis; Psoriasis; Lupus Erythematosus, Systemic; Liver Cirrhosis, Biliary; Multiple Sclerosis; Sjogren's Syndrome; Pouchitis; Ichthyosis; Inflammatory Bowel Diseases; Type I Leukocyte Adhesion Defect; Dermatomyositis; Non-radiographic axial spondyloarthritis; Diabetes Mellitus, Type 1; Polymyositis | Details |
| Ustekinumab biosimilar (Alvotech) | AVT04; AVT-04 | Approved | Alvotech hf | Jamteki, Uzpruvo, SELARSDI™ | Japan | Psoriasis | Fuji Pharma Co Ltd | 2023-09-25 | Psoriasis; Arthritis, Psoriatic; Plaque psoriasis; Crohn Disease | Details |
| Ustekinumab biosimilar(Amgen) | ABP-654 | Approved | Amgen Inc | Wezlana, WEZLANA | United States | Crohn Disease; Plaque psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative | Amgen Inc | 2023-10-31 | Psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative; Crohn Disease; Plaque psoriasis | Details |
| Thalidomide | NSC-66847; NSC-527179; K-17; VP-02; FPF-300; FPF300 | Approved | Celgene Corp | Talizer, Thalidomide Celgene, Thalidomide Pharmion, Synovir, Thalomid, Thaled | Mainland China | Leprosy, Lepromatous; Multiple Myeloma | Changzhou Pharmaceutical Factory | 1982-01-01 | HIV Wasting Syndrome; Angiodysplasia; Primary Myelofibrosis; Neuroectodermal Tumors, Primitive; Prostatitis; Colorectal Neoplasms; Osteosarcoma; Lymphoma, Mantle-Cell; Sarcoma, Ewing; Retinoblastoma; Erythema Nodosum; Drug Resistant Epilepsy; Xerostomia; Sarcoma; Pancreatitis, Chronic; Adenocarcinoma, Clear Cell; Lymphoma, Follicular; Arachnoiditis; Carcinoma, Adenosquamous; Gastrointestinal Hemorrhage; Cholangitis, Sclerosing; Prostatic Neoplasms; Pelvic Pain; Neoplasm Metastasis; Stomatitis; Burning Mouth Syndrome; Mycobacterium avium-intracellulare Infection; Amyotrophic Lateral Sclerosis; Melanoma; Myelodysplastic-Myeloproliferative Diseases; Carcinoma, Hepatocellular; Leukemia, Lymphocytic, Chronic, B-Cell; Vascular Malformations; Tuberculosis; Appendiceal Neoplasms; Lymphoma, Non-Hodgkin; Uterine Neoplasms; Anemia, Sideroblastic; Glioma; Leprosy, Lepromatous; Endometrial Neoplasms; Lung Neoplasms; Waldenstrom Macroglobulinemia; Kidney Neoplasms; Thalassemia; Carcinoid Tumor; Lupus Erythematosus, Discoid | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Ustekinumab biosimilar (Bio-Thera ) | BAT-2206 | Phase 3 Clinical | Bio-Thera Solutions Ltd | Autoimmune Diseases; Psoriasis; Colitis, Ulcerative; Arthritis, Psoriatic; Plaque psoriasis; Crohn Disease | Details |
| Ustekinumab biosimilar(Formycon) | FYB-202 | Phase 3 Clinical | Psoriasis | Details | |
| Lisofylline | BL-194; CT-1501R; A-802710 | Phase 3 Clinical | Cti Biopharma Corp | Diabetes Mellitus, Type 1; Rejection of organ transplantation | Details |
| Ustekinumab Biosimilar (qyuns) | QX-001-S; HDM-3001; QX-001S | Phase 3 Clinical | Qyuns Therapeutics Co Ltd | Psoriasis; Plaque psoriasis | Details |
| Ustekinumab Biosimilar(Celltrion) | CT-P43 | Phase 3 Clinical | Celltrion Inc | Psoriasis | Details |
| Ustekinumab biosimilar(Biocon) | Phase 3 Clinical | Biocon Biologics Ltd | Psoriasis | Details | |
| Ustekinumab biosimilar(CSPC Pharma) | SYSA-1902 | Phase 3 Clinical | Jushi Biopharmaceutical Co Ltd | Psoriasis; Plaque psoriasis | Details |
| Apilimod mesilate | AIT-101; STA-5326-mesylate; LAM-002; STA-5326; LAM-002A | Phase 2 Clinical | Synta Pharmaceuticals Corp, Lam | Coronavirus Disease 2019 (COVID-19); Arthritis, Rheumatoid; Common Variable Immunodeficiency; Crohn Disease; Amyotrophic Lateral Sclerosis | Details |
| NSC-733972 | M-032; M032-H SV-1; NSC-733972 | Phase 2 Clinical | University Of Alabama At Birmingham | Glioblastoma; Gliosarcoma; Astrocytoma | Details |
| Tavokinogene telseplasmid (OncoSec Medical) | pIL-12; DNA IL-12 | Phase 2 Clinical | Inovio Pharmaceuticals Inc | Triple Negative Breast Neoplasms; Melanoma; Carcinoma, Hepatocellular; Mycosis Fungoides; Lung Neoplasms; Lymphoma, T-Cell, Cutaneous; Colorectal Neoplasms; Breast Neoplasms; Hepatitis B; Prostatic Neoplasms; Small Cell Lung Carcinoma; Hemorrhagic Fever, Ebola; Pancreatic Neoplasms; Neoplasms; Carcinoma, Merkel Cell; Stomach Neoplasms; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; HIV Infections; Head and Neck Neoplasms; Ovarian Neoplasms | Details |
| INO-9012/Human papillomavirus vaccine | INO-3112; INO-9012/VGX-3100; VGX-3100/INO-9012 | Phase 2 Clinical | University Of Pennsylvania, Astrazeneca Plc, Inovio Pharmaceuticals Inc | Squamous Cell Carcinoma of Head and Neck; Anus Neoplasms; Vaginal Neoplasms; Papillomavirus Infections; Neoplasms; Carcinoma, Transitional Cell; Vulvar Neoplasms; Oropharyngeal Neoplasms; Penile Neoplasms; Uterine Cervical Neoplasms | Details |
| T-3011 | T-3 (ImmVira Pharma); MVR-T3011; T3011; MVR-T3011 IT; MVR-T3011 IV; B015; B-015 | Phase 2 Clinical | Immvira Co Ltd | Breast Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Melanoma; Carcinoma, Squamous Cell; Lymphoma; Endometrial Neoplasms; Lung Neoplasms; Colorectal Neoplasms; Sarcoma; Liver Neoplasms; Ascites; Mesothelioma; Small Cell Lung Carcinoma; Skin Neoplasms; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Head and Neck Neoplasms; Solid tumours; Ovarian Neoplasms | Details |
| PDS-0301 | M-9241; MSB-0010360; MSB0010360; PDS-0301 | Phase 2 Clinical | Emd Serono Inc, Merck Serono, National Cancer Institute | Prostatic Neoplasms; Sarcoma, Kaposi; Uterine Cervical Neoplasms; Urogenital Neoplasms; Bile Duct Neoplasms; Colorectal Neoplasms; Oropharyngeal Neoplasms; Cholangiocarcinoma; Vulvar Neoplasms; Intestinal Neoplasms; Breast Neoplasms; Urinary Bladder Neoplasms; Prostatic Neoplasms, Castration-Resistant; Pancreatic Neoplasms; Papillomavirus Infections; Rectal Neoplasms; Carcinoma; Anus Neoplasms | Details |
| STX-001 | STX-001 | Phase 2 Clinical | Massachusetts Institute Of Technology | Solid tumours | Details |
| DF-6002 | DF-6002 | Phase 2 Clinical | Dragonfly Therapeutics Llc | Solid tumours; Neoplasms | Details |
| Recombinant Human nsIL12 Oncolytic Adenovirus (Beijing Haute Biotechnology) | BioTTT-001; BioTTT001 | Phase 2 Clinical | Beijing Bio-Targeting Therapeutics Technology Co Ltd | Solid tumours; Stomach Neoplasms; Neoplasms; Colorectal Neoplasms | Details |
| Recombinant Human IL12/15-PDL1B Herpes Simplex Type I Oncolytic Virus(CNBG) | VG-161 | Phase 2 Clinical | Cnbg-Virogin Biotech (Shanghai) Ltd | Solid tumours; Liver Neoplasms; Biliary Tract Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Neoplasms; Pancreatic Neoplasms; Sarcoma; Cholangiocarcinoma; Carcinoma, Hepatocellular | Details |
| Rocakinogene sifuplasmid | INO-9012 | Phase 2 Clinical | Inovio Pharmaceuticals Inc | Urogenital Abnormalities; Carcinoma, Hepatocellular; Precancerous Conditions; Lung Neoplasms; Vulvar Diseases; Hepatitis C; Colorectal Neoplasms; Squamous Intraepithelial Lesions of the Cervix; Breast Neoplasms; Prostatic Neoplasms; Small Cell Lung Carcinoma; Neoplasms; Carcinoma, Transitional Cell; Liver Neoplasms; Neoplasms, Glandular and Epithelial; Papillomavirus Infections; Pancreatic Neoplasms; Glioblastoma; Esophageal Neoplasms; Stomach Neoplasms; Hemorrhagic Fever, Ebola; Hepatitis, Chronic; Head and Neck Neoplasms; HIV Infections; Ovarian Neoplasms | Details |
| Veledimex | INXN-1001; AD-1001 | Phase 1 Clinical | Ziopharm Oncology Inc | Glioblastoma; Brain Neoplasms; Breast Neoplasms; Astrocytoma; Diffuse Intrinsic Pontine Glioma; Melanoma | Details |
| XTX-301 | XTX-301 | Phase 1 Clinical | Solid tumours; Neoplasms | Details | |
| VG-201 | VG-201 | Phase 1 Clinical | ViroGin Biotech Ltd | Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| VG-203 | VG-203; VG2062; VG-2062 | Phase 1 Clinical | ViroGin Biotech Ltd, Shanghai Funuojian Biotechnology Co Ltd, Virogin Biotech (Shenzhen) Ltd, Fenojian Biotechnology (Nantong) Co Ltd | Ovarian Neoplasms; Solid tumours; Carcinoma, Renal Cell; Breast Neoplasms | Details |
| MVR-C5252 | C-5252; MVR-C5252 | Phase 1 Clinical | Immvira Co Ltd | Solid tumours; Ganglioglioma; Glioblastoma; Brain Neoplasms; Glioma | Details |
| WTX-330 | WTX-330 | Phase 1 Clinical | Werewolf Therapeutics Inc | Solid tumours; Neoplasms; Lymphoma, Non-Hodgkin | Details |
| WTX-124 | WTX-124 | Phase 1 Clinical | Werewolf Therapeutics Inc | Solid tumours; Neoplasm Metastasis | Details |
| IL-12 DNA(NIAID) | Phase 1 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid) | HIV Infections | Details | |
| SW-0715 | SW-0715 | Phase 1 Clinical | Stemirna Therapeutics | Solid tumours | Details |
| KGX-101 | KGX-101; KGX101; KGX-101(IL-12-Fc融合蛋白); KGX-101(改造优化的细胞因子IL12前药分子) | Phase 1 Clinical | Shanghai KangaBio Co Ltd | Solid tumours; Neoplasms | Details |
| Ustekinumab Biosimilar (NeuClone/Serum Institute of India) | Phase 1 Clinical | Neuclone, Serum Institute Of India Ltd | Autoimmune Diseases | Details | |
| TG-6050 | TG-6050 | Phase 1 Clinical | Transgene Sa | Carcinoma, Non-Small-Cell Lung | Details |
| Ustekinumab biosimilar(Rani Therapeutics) | RT-111 | Phase 1 Clinical | Rani Therapeutics Llc | Psoriasis | Details |
| ABOD-2011 | ABOD-2011 | Phase 1 Clinical | Cancer Hospital Of Chinese Academy Of Medical Sciences | Solid tumours | Details |
| JCXH-211 | JCXH-211 | Phase 1 Clinical | Jiachen Xihai(Hangzhou)Biotechnology Co Ltd | Solid tumours; Skin Neoplasms | Details |
| EGFR IL12 CART | Phase 1 Clinical | Shenzhen Prekin Biopharmaceutical Co Ltd | Colorectal Neoplasms | Details | |
| XmAb-662 | XmAb-662 | Phase 1 Clinical | Xencor Inc | Solid tumours; Neoplasms | Details |
| MEDI-1191 | MEDI-1191; MEDI1191 | Phase 1 Clinical | Moderna Inc | Solid tumours; Neoplasms | Details |
| Ustekinumab biosimilar (BioFactura) | BFI-751 | Phase 1 Clinical | BioFactura Australia Pty Ltd | Psoriasis | Details |
| MEDI-9253 | Phase 1 Clinical | Astrazeneca Plc | Solid tumours; Neoplasms | Details | |
| INXN-2001 | Ad-IL-12; ZIN-ATI-001; Ad-RTS-hIL-12; Ad-RTS-mIL-12; INXN-2001; Ad-RTS-IL-12; INXN-2001 (Ad-RTS-IL-12) | Phase 1 Clinical | Ziopharm Oncology Inc | Glioblastoma; Breast Neoplasms; Brain Neoplasms; Diffuse Intrinsic Pontine Glioma; Astrocytoma; Melanoma | Details |
| Ustekinumab biosimilar (Bio-Thera ) | BAT-2206 | Phase 3 Clinical | Bio-Thera Solutions Ltd | Autoimmune Diseases; Psoriasis; Colitis, Ulcerative; Arthritis, Psoriatic; Plaque psoriasis; Crohn Disease | Details |
| Ustekinumab biosimilar(Formycon) | FYB-202 | Phase 3 Clinical | Psoriasis | Details | |
| Lisofylline | BL-194; CT-1501R; A-802710 | Phase 3 Clinical | Cti Biopharma Corp | Diabetes Mellitus, Type 1; Rejection of organ transplantation | Details |
| Ustekinumab Biosimilar (qyuns) | QX-001-S; HDM-3001; QX-001S | Phase 3 Clinical | Qyuns Therapeutics Co Ltd | Psoriasis; Plaque psoriasis | Details |
| Ustekinumab Biosimilar(Celltrion) | CT-P43 | Phase 3 Clinical | Celltrion Inc | Psoriasis | Details |
| Ustekinumab biosimilar(Biocon) | Phase 3 Clinical | Biocon Biologics Ltd | Psoriasis | Details | |
| Ustekinumab biosimilar(CSPC Pharma) | SYSA-1902 | Phase 3 Clinical | Jushi Biopharmaceutical Co Ltd | Psoriasis; Plaque psoriasis | Details |
| Apilimod mesilate | AIT-101; STA-5326-mesylate; LAM-002; STA-5326; LAM-002A | Phase 2 Clinical | Synta Pharmaceuticals Corp, Lam | Coronavirus Disease 2019 (COVID-19); Arthritis, Rheumatoid; Common Variable Immunodeficiency; Crohn Disease; Amyotrophic Lateral Sclerosis | Details |
| NSC-733972 | M-032; M032-H SV-1; NSC-733972 | Phase 2 Clinical | University Of Alabama At Birmingham | Glioblastoma; Gliosarcoma; Astrocytoma | Details |
| Tavokinogene telseplasmid (OncoSec Medical) | pIL-12; DNA IL-12 | Phase 2 Clinical | Inovio Pharmaceuticals Inc | Triple Negative Breast Neoplasms; Melanoma; Carcinoma, Hepatocellular; Mycosis Fungoides; Lung Neoplasms; Lymphoma, T-Cell, Cutaneous; Colorectal Neoplasms; Breast Neoplasms; Hepatitis B; Prostatic Neoplasms; Small Cell Lung Carcinoma; Hemorrhagic Fever, Ebola; Pancreatic Neoplasms; Neoplasms; Carcinoma, Merkel Cell; Stomach Neoplasms; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; HIV Infections; Head and Neck Neoplasms; Ovarian Neoplasms | Details |
| INO-9012/Human papillomavirus vaccine | INO-3112; INO-9012/VGX-3100; VGX-3100/INO-9012 | Phase 2 Clinical | University Of Pennsylvania, Astrazeneca Plc, Inovio Pharmaceuticals Inc | Squamous Cell Carcinoma of Head and Neck; Anus Neoplasms; Vaginal Neoplasms; Papillomavirus Infections; Neoplasms; Carcinoma, Transitional Cell; Vulvar Neoplasms; Oropharyngeal Neoplasms; Penile Neoplasms; Uterine Cervical Neoplasms | Details |
| T-3011 | T-3 (ImmVira Pharma); MVR-T3011; T3011; MVR-T3011 IT; MVR-T3011 IV; B015; B-015 | Phase 2 Clinical | Immvira Co Ltd | Breast Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Melanoma; Carcinoma, Squamous Cell; Lymphoma; Endometrial Neoplasms; Lung Neoplasms; Colorectal Neoplasms; Sarcoma; Liver Neoplasms; Ascites; Mesothelioma; Small Cell Lung Carcinoma; Skin Neoplasms; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Head and Neck Neoplasms; Solid tumours; Ovarian Neoplasms | Details |
| PDS-0301 | M-9241; MSB-0010360; MSB0010360; PDS-0301 | Phase 2 Clinical | Emd Serono Inc, Merck Serono, National Cancer Institute | Prostatic Neoplasms; Sarcoma, Kaposi; Uterine Cervical Neoplasms; Urogenital Neoplasms; Bile Duct Neoplasms; Colorectal Neoplasms; Oropharyngeal Neoplasms; Cholangiocarcinoma; Vulvar Neoplasms; Intestinal Neoplasms; Breast Neoplasms; Urinary Bladder Neoplasms; Prostatic Neoplasms, Castration-Resistant; Pancreatic Neoplasms; Papillomavirus Infections; Rectal Neoplasms; Carcinoma; Anus Neoplasms | Details |
| STX-001 | STX-001 | Phase 2 Clinical | Massachusetts Institute Of Technology | Solid tumours | Details |
| DF-6002 | DF-6002 | Phase 2 Clinical | Dragonfly Therapeutics Llc | Solid tumours; Neoplasms | Details |
| Recombinant Human nsIL12 Oncolytic Adenovirus (Beijing Haute Biotechnology) | BioTTT-001; BioTTT001 | Phase 2 Clinical | Beijing Bio-Targeting Therapeutics Technology Co Ltd | Solid tumours; Stomach Neoplasms; Neoplasms; Colorectal Neoplasms | Details |
| Recombinant Human IL12/15-PDL1B Herpes Simplex Type I Oncolytic Virus(CNBG) | VG-161 | Phase 2 Clinical | Cnbg-Virogin Biotech (Shanghai) Ltd | Solid tumours; Liver Neoplasms; Biliary Tract Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Neoplasms; Pancreatic Neoplasms; Sarcoma; Cholangiocarcinoma; Carcinoma, Hepatocellular | Details |
| Rocakinogene sifuplasmid | INO-9012 | Phase 2 Clinical | Inovio Pharmaceuticals Inc | Urogenital Abnormalities; Carcinoma, Hepatocellular; Precancerous Conditions; Lung Neoplasms; Vulvar Diseases; Hepatitis C; Colorectal Neoplasms; Squamous Intraepithelial Lesions of the Cervix; Breast Neoplasms; Prostatic Neoplasms; Small Cell Lung Carcinoma; Neoplasms; Carcinoma, Transitional Cell; Liver Neoplasms; Neoplasms, Glandular and Epithelial; Papillomavirus Infections; Pancreatic Neoplasms; Glioblastoma; Esophageal Neoplasms; Stomach Neoplasms; Hemorrhagic Fever, Ebola; Hepatitis, Chronic; Head and Neck Neoplasms; HIV Infections; Ovarian Neoplasms | Details |
| Veledimex | INXN-1001; AD-1001 | Phase 1 Clinical | Ziopharm Oncology Inc | Glioblastoma; Brain Neoplasms; Breast Neoplasms; Astrocytoma; Diffuse Intrinsic Pontine Glioma; Melanoma | Details |
| XTX-301 | XTX-301 | Phase 1 Clinical | Solid tumours; Neoplasms | Details | |
| VG-201 | VG-201 | Phase 1 Clinical | ViroGin Biotech Ltd | Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| VG-203 | VG-203; VG2062; VG-2062 | Phase 1 Clinical | ViroGin Biotech Ltd, Shanghai Funuojian Biotechnology Co Ltd, Virogin Biotech (Shenzhen) Ltd, Fenojian Biotechnology (Nantong) Co Ltd | Ovarian Neoplasms; Solid tumours; Carcinoma, Renal Cell; Breast Neoplasms | Details |
| MVR-C5252 | C-5252; MVR-C5252 | Phase 1 Clinical | Immvira Co Ltd | Solid tumours; Ganglioglioma; Glioblastoma; Brain Neoplasms; Glioma | Details |
| WTX-330 | WTX-330 | Phase 1 Clinical | Werewolf Therapeutics Inc | Solid tumours; Neoplasms; Lymphoma, Non-Hodgkin | Details |
| WTX-124 | WTX-124 | Phase 1 Clinical | Werewolf Therapeutics Inc | Solid tumours; Neoplasm Metastasis | Details |
| IL-12 DNA(NIAID) | Phase 1 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid) | HIV Infections | Details | |
| SW-0715 | SW-0715 | Phase 1 Clinical | Stemirna Therapeutics | Solid tumours | Details |
| KGX-101 | KGX-101; KGX101; KGX-101(IL-12-Fc融合蛋白); KGX-101(改造优化的细胞因子IL12前药分子) | Phase 1 Clinical | Shanghai KangaBio Co Ltd | Solid tumours; Neoplasms | Details |
| Ustekinumab Biosimilar (NeuClone/Serum Institute of India) | Phase 1 Clinical | Neuclone, Serum Institute Of India Ltd | Autoimmune Diseases | Details | |
| TG-6050 | TG-6050 | Phase 1 Clinical | Transgene Sa | Carcinoma, Non-Small-Cell Lung | Details |
| Ustekinumab biosimilar(Rani Therapeutics) | RT-111 | Phase 1 Clinical | Rani Therapeutics Llc | Psoriasis | Details |
| ABOD-2011 | ABOD-2011 | Phase 1 Clinical | Cancer Hospital Of Chinese Academy Of Medical Sciences | Solid tumours | Details |
| JCXH-211 | JCXH-211 | Phase 1 Clinical | Jiachen Xihai(Hangzhou)Biotechnology Co Ltd | Solid tumours; Skin Neoplasms | Details |
| EGFR IL12 CART | Phase 1 Clinical | Shenzhen Prekin Biopharmaceutical Co Ltd | Colorectal Neoplasms | Details | |
| XmAb-662 | XmAb-662 | Phase 1 Clinical | Xencor Inc | Solid tumours; Neoplasms | Details |
| MEDI-1191 | MEDI-1191; MEDI1191 | Phase 1 Clinical | Moderna Inc | Solid tumours; Neoplasms | Details |
| Ustekinumab biosimilar (BioFactura) | BFI-751 | Phase 1 Clinical | BioFactura Australia Pty Ltd | Psoriasis | Details |
| MEDI-9253 | Phase 1 Clinical | Astrazeneca Plc | Solid tumours; Neoplasms | Details | |
| INXN-2001 | Ad-IL-12; ZIN-ATI-001; Ad-RTS-hIL-12; Ad-RTS-mIL-12; INXN-2001; Ad-RTS-IL-12; INXN-2001 (Ad-RTS-IL-12) | Phase 1 Clinical | Ziopharm Oncology Inc | Glioblastoma; Breast Neoplasms; Brain Neoplasms; Diffuse Intrinsic Pontine Glioma; Astrocytoma; Melanoma | Details |
This web search service is supported by Google Inc.
