 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
| CD4-H82E3 | Human | Biotinylated Human CD34 Protein, His,Avitag™ (MALS verified) |  |
|
|
| CD4-H52H9 | Human | Human CD34 Protein, His Tag |  |

|

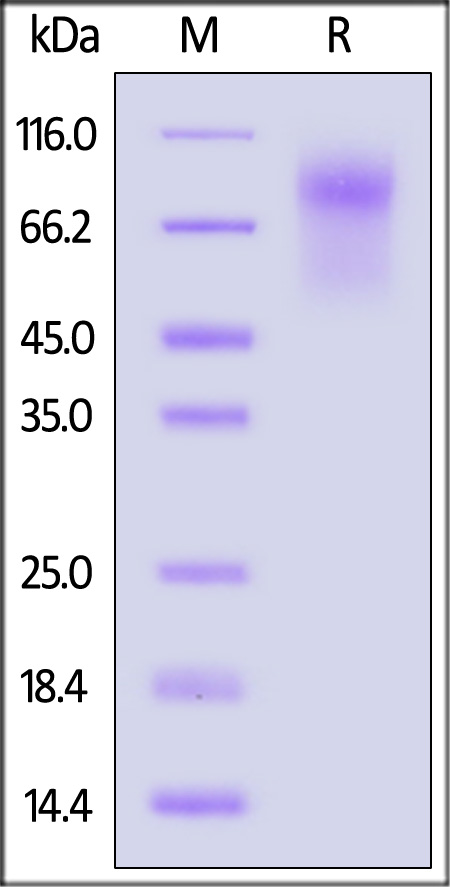
The purity of Biotinylated Human CD34 Protein, His,Avitag (Cat. No. CD4-H82E3) is more than 90% and the molecular weight of this protein is around 55-75 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Anti-CD34 mab-sirolimus (OrbusNeich) | Approved | Orbusneich | Coronary Restenosis | Orbusneich | 2013-01-01 | Coronary Artery Disease; Coronary Restenosis | Details | |||
| Anti-CD34 mab-sirolimus (OrbusNeich) | Approved | Orbusneich | Coronary Restenosis | Orbusneich | 2013-01-01 | Coronary Artery Disease; Coronary Restenosis | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| CLBS-12 | CLBS-12 | Phase 2 Clinical | Caladrius Biosciences Inc | Atherosclerosis; Thromboangiitis Obliterans; Ischemia | Details |
| Retroviral ADA-transduced CD34+ cells (NHGRI/UCLA) | Phase 2 Clinical | Ucla Biomedical Library | Adenosine deaminase deficiency | Details | |
| CD34+ bone marrow stem cells (University of California) | Phase 2 Clinical | The University Of California Davis | Retinitis Pigmentosa; Diabetic Retinopathy; Macular Degeneration; Retinal Vein Occlusion | Details | |
| GSK-2696277 | GSK-2696277; OTL-300 | Phase 2 Clinical | Glaxosmithkline Plc, Ospedale San Raffaele, Fondazione Telethon | beta-Thalassemia | Details |
| Autologous peripheral blood stem cell therapy (CellProthera) | Phase 2 Clinical | Cellprothera | Myocardial Infarction | Details | |
| CD34 stem cell therapy (LA Childrens Hospital) | Phase 2 Clinical | Children'S Hospital Of Los Angeles | HIV Infections; Heart Failure | Details | |
| Stem cell therapeutics (ExCellThera) | ECT-002; ECT-003; ECT-005; UM171 + UM092; UM171 + UM390 | Phase 2 Clinical | Excellthera Inc | Hematologic Neoplasms; Multiple Myeloma; Leukemia, Myeloid, Acute | Details |
| Lentiviral vector transduced CD34+ cells Therapy | Phase 2 Clinical | Great Ormond Street Hospital For Children Nhs Foundation Trust | Granulomatous Disease, Chronic | Details | |
| Cord blood stem cells(Tatcitus Therapeutics/Mount Sinai Health System) | HSC-100 | Phase 1 Clinical | Icahn School Of Medicine At Mount Sinai, Tatcitus Therapeutics | Hematologic Neoplasms; Leukemia; Hodgkin Disease; Myelodysplastic Syndromes; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details |
| shRNA-Modified CD34 Cell Therapy | KL-AI20 | Phase 1 Clinical | Kanglin Biotech (Hangzhou) Co Ltd | HIV Infections | Details |
| Alpha-galactosidase A stem cell therapy (University Health Network/Ozmosis) | Phase 1 Clinical | University Health Network | Fabry Disease | Details | |
| Autologous CD34+ Cells Therapy(Genmedicn) | GMCN-508A | Phase 1 Clinical | Zhongji Zhiyao (Nanjing) Biotechnology Co Ltd, First Affiliated Hospital Of Guangxi Medical University | alpha-Thalassemia | Details |
| Donor-derived CD34+ HSC with CRISPR/Cas9-mediated CD33 deletion(German Cancer Research Center) | Phase 1 Clinical | German Cancer Research Center (Dkfz) | Leukemia, Myeloid, Acute | Details | |
| CLBS-12 | CLBS-12 | Phase 2 Clinical | Caladrius Biosciences Inc | Atherosclerosis; Thromboangiitis Obliterans; Ischemia | Details |
| Retroviral ADA-transduced CD34+ cells (NHGRI/UCLA) | Phase 2 Clinical | Ucla Biomedical Library | Adenosine deaminase deficiency | Details | |
| CD34+ bone marrow stem cells (University of California) | Phase 2 Clinical | The University Of California Davis | Retinitis Pigmentosa; Diabetic Retinopathy; Macular Degeneration; Retinal Vein Occlusion | Details | |
| GSK-2696277 | GSK-2696277; OTL-300 | Phase 2 Clinical | Glaxosmithkline Plc, Ospedale San Raffaele, Fondazione Telethon | beta-Thalassemia | Details |
| Autologous peripheral blood stem cell therapy (CellProthera) | Phase 2 Clinical | Cellprothera | Myocardial Infarction | Details | |
| CD34 stem cell therapy (LA Childrens Hospital) | Phase 2 Clinical | Children'S Hospital Of Los Angeles | HIV Infections; Heart Failure | Details | |
| Stem cell therapeutics (ExCellThera) | ECT-002; ECT-003; ECT-005; UM171 + UM092; UM171 + UM390 | Phase 2 Clinical | Excellthera Inc | Hematologic Neoplasms; Multiple Myeloma; Leukemia, Myeloid, Acute | Details |
| Lentiviral vector transduced CD34+ cells Therapy | Phase 2 Clinical | Great Ormond Street Hospital For Children Nhs Foundation Trust | Granulomatous Disease, Chronic | Details | |
| Cord blood stem cells(Tatcitus Therapeutics/Mount Sinai Health System) | HSC-100 | Phase 1 Clinical | Icahn School Of Medicine At Mount Sinai, Tatcitus Therapeutics | Hematologic Neoplasms; Leukemia; Hodgkin Disease; Myelodysplastic Syndromes; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details |
| shRNA-Modified CD34 Cell Therapy | KL-AI20 | Phase 1 Clinical | Kanglin Biotech (Hangzhou) Co Ltd | HIV Infections | Details |
| Alpha-galactosidase A stem cell therapy (University Health Network/Ozmosis) | Phase 1 Clinical | University Health Network | Fabry Disease | Details | |
| Autologous CD34+ Cells Therapy(Genmedicn) | GMCN-508A | Phase 1 Clinical | Zhongji Zhiyao (Nanjing) Biotechnology Co Ltd, First Affiliated Hospital Of Guangxi Medical University | alpha-Thalassemia | Details |
| Donor-derived CD34+ HSC with CRISPR/Cas9-mediated CD33 deletion(German Cancer Research Center) | Phase 1 Clinical | German Cancer Research Center (Dkfz) | Leukemia, Myeloid, Acute | Details |
This web search service is supported by Google Inc.
