 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
| CEA-B039 | Human | ClinMax™ Human Soluble CD38 ELISA Kit | |||
| CD8-H8214 | Human | Biotinylated Human CD38 Protein, Avitag™ |  |
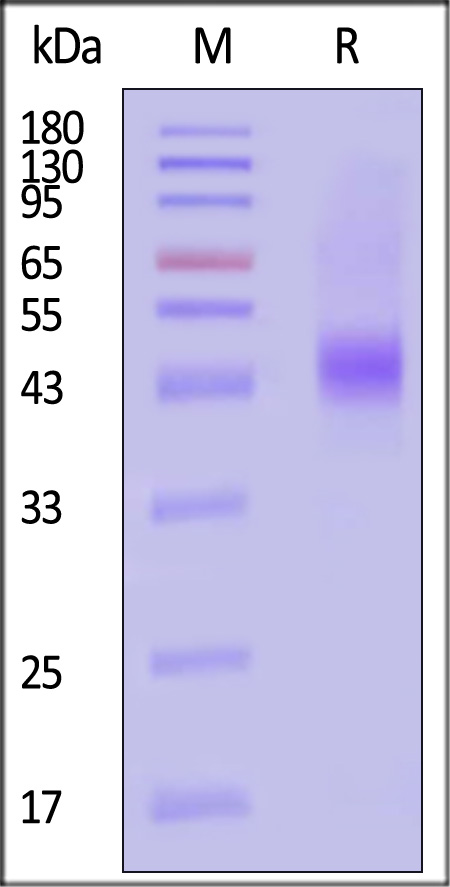
|
|
| CD8-H82F5 | Human | Biotinylated Human CD38 Protein, Fc,Avitag™ (MALS verified) |  |
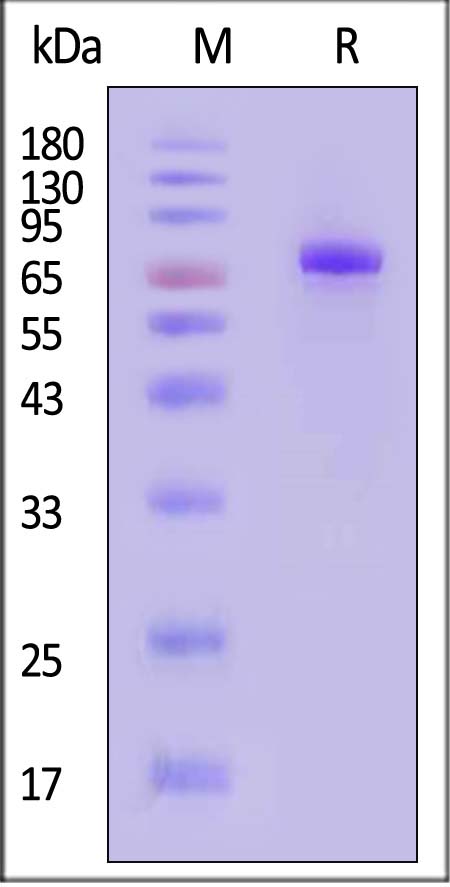
|
|
| CD8-HF255 | Human | FITC-Labeled Human CD38 Protein, Fc Tag |  |
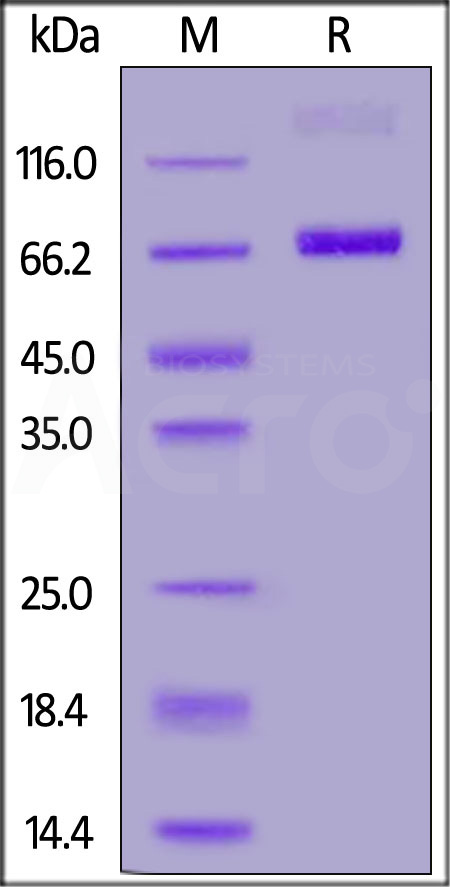
|
|
| CD8-HF2H5 | Human | FITC-Labeled Human CD38 Protein, His Tag |  |
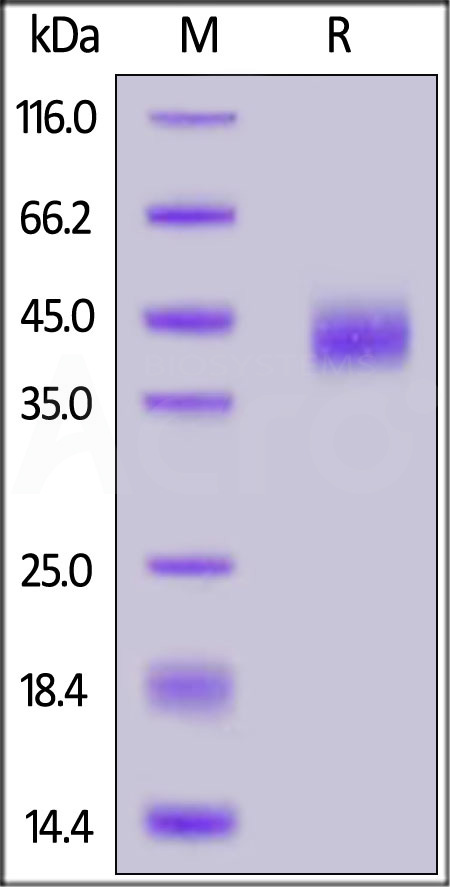
|
|
| MBC-K010 | Human | Human CD38-coupled Magnetic Beads | |||
| CD8-M5223 | Mouse | Mouse CD38 Protein, His Tag |  |
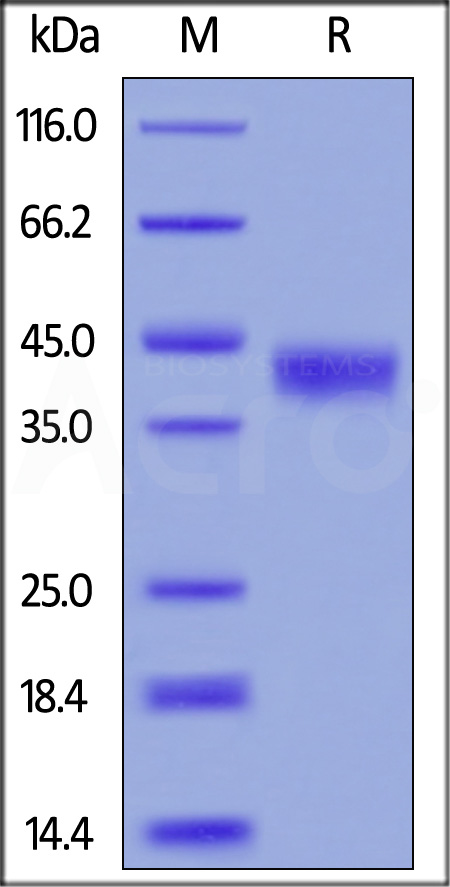
|
|
| CD8-C5223 | Cynomolgus | Cynomolgus CD38 Protein, His Tag |  |
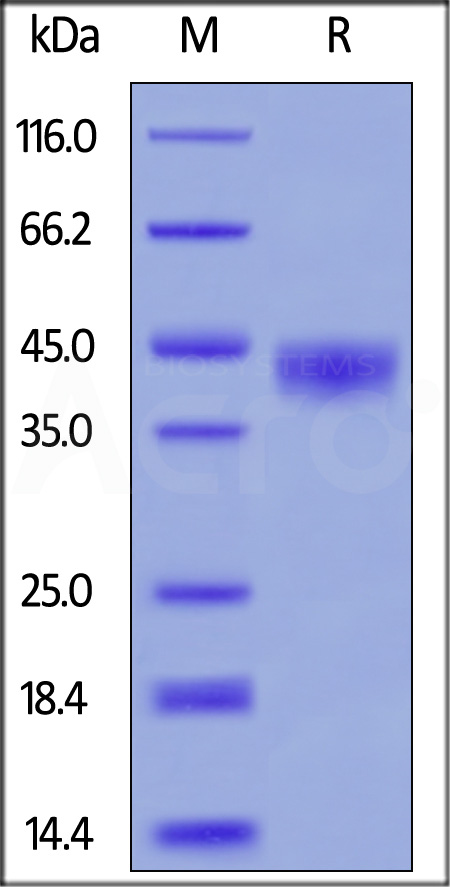
|
|
| CD8-H5252 | Human | Human CD38 Protein, Llama IgG2b Fc Tag, low endotoxin |  |
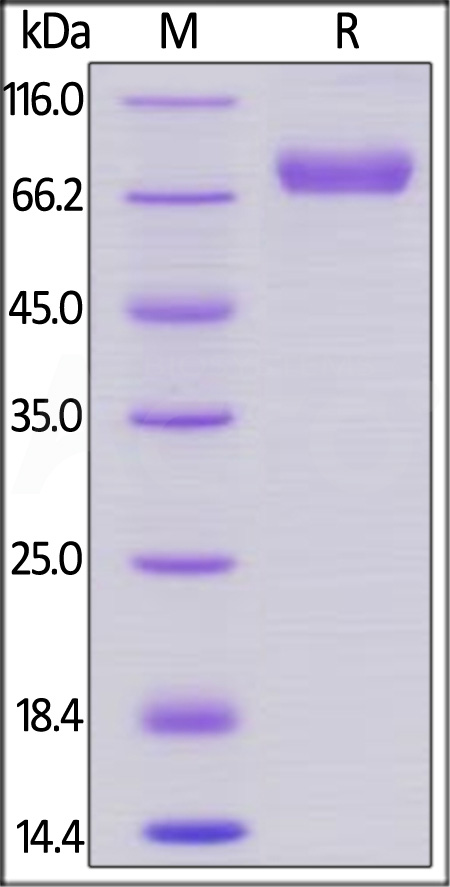
|
|
| CD8-H5253 | Human | Human CD38 Protein, Mouse IgG2a Fc Tag, low endotoxin |  |
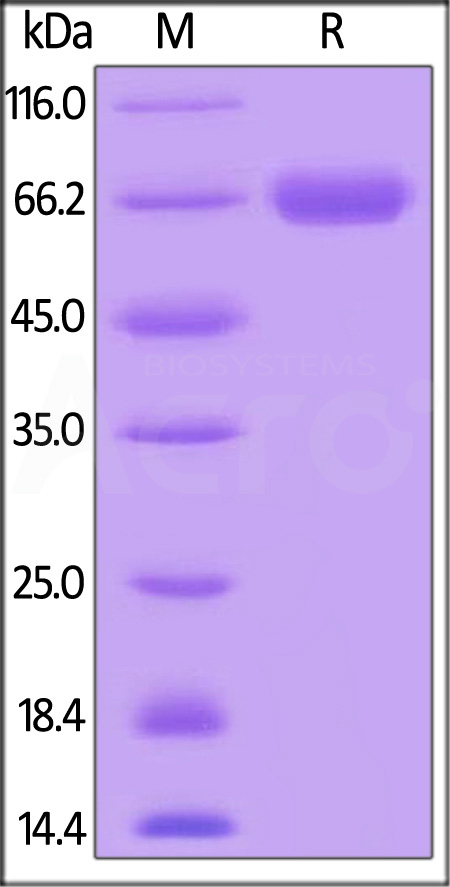
|
|
| CD8-H5255 | Human | Human CD38 Protein, Fc Tag (MALS verified) |  |
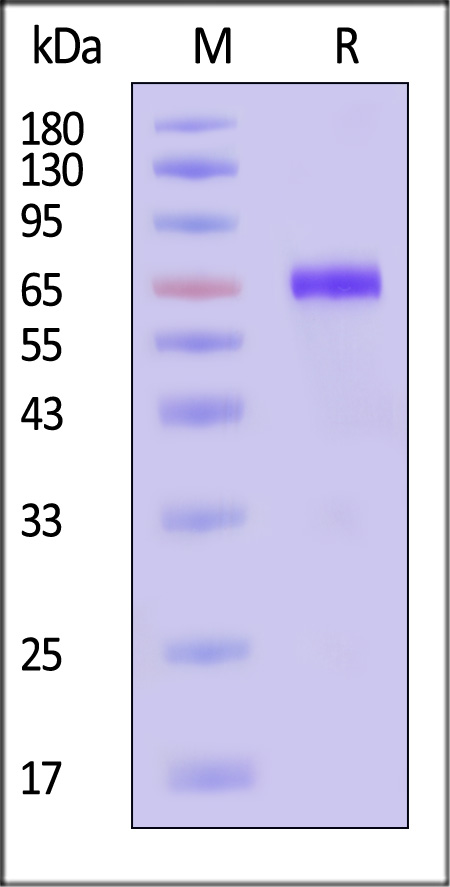
|
|
| CD8-H82E7 | Human | Biotinylated Human CD38 Protein, Avitag™,His Tag |  |
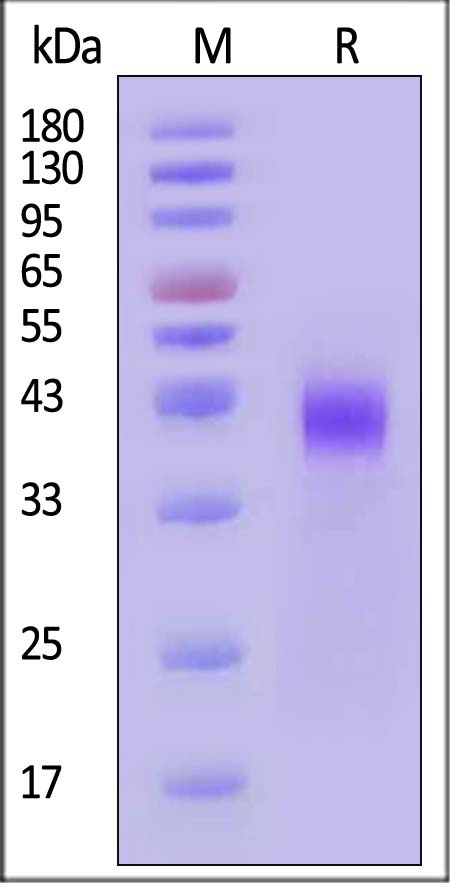
|
|
| CD8-H5224 | Human | Human CD38 Protein, His Tag (MALS verified) |  |

|
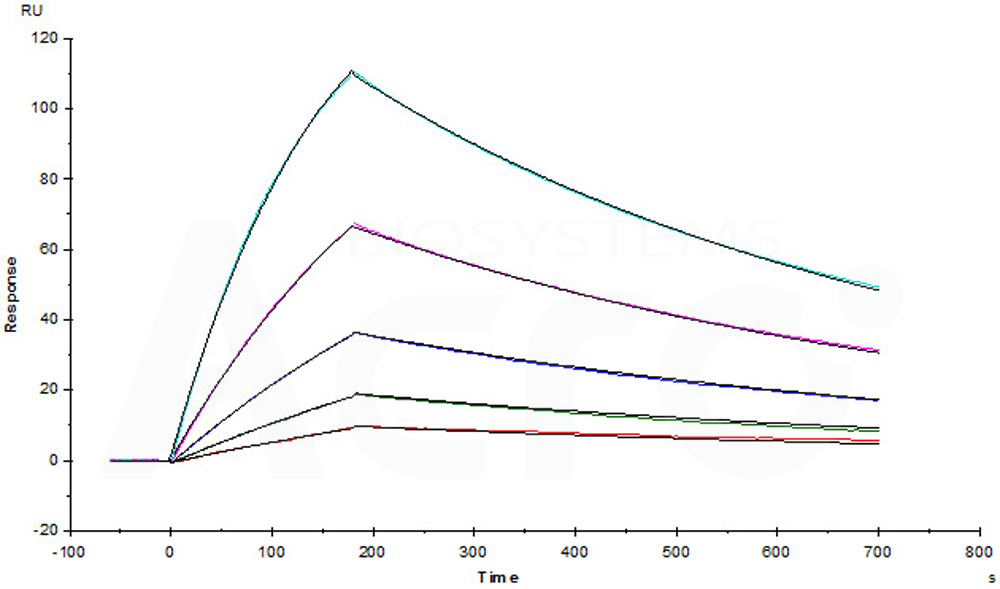
Anti-CD38 mAb (Human IgG1) captured on CM5 chip via anti-human IgG Fc antibodies surface, can bind Human CD38, His Tag (Cat. No. CD8-H5224) with an affinity constant of 4.98 nM as determined in a SPR assay (Biacore T200) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Daratumumab/Hyaluronidase-fihj | Approved | Johnson & Johnson Innovative Medicine | Darzalex Faspro, Darzquro | United States | Multiple Myeloma | Janssen Biotech Inc | 2020-05-01 | Peripheral Nervous System Diseases; Multiple Myeloma; Monoclonal Gammopathy of Undetermined Significance; Rejection of organ transplantation; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Amyloidosis; Rejection in heart transplantation | Details | |
| Isatuximab | SAR-650984 | Approved | Immunogen Inc | Sarclisa | United States | Multiple Myeloma | Sanofi-Aventis U.S. Llc | 2020-03-02 | Anemia, Aplastic; Leukemia, T-Cell; Carcinoma, Non-Small-Cell Lung; Macroglossia; Leukemia, Myeloid, Acute; Lymphoma, Non-Hodgkin; Lymphoma; Nausea; Hepatomegaly; Gastrointestinal Hemorrhage; Satiety Response; Diarrhea; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Hematologic Neoplasms; Anemia, Hemolytic, Autoimmune; Prostatic Neoplasms; Multiple Myeloma; Immunoglobulin Light-chain Amyloidosis; Purpura; Neoplasms; Smoldering Multiple Myeloma; Hodgkin Disease; Lymphadenopathy; Paraproteinemias; Constipation; Goiter, Nodular | Details |
| Daratumumab | JNJ-54767414 | Approved | Johnson & Johnson Innovative Medicine | Darzalex, 兆珂速, 兆珂 | United States | Multiple Myeloma | Janssen Biotech Inc | 2015-11-16 | Prostatic Neoplasms; POEMS Syndrome; Autoimmune Diseases of the Nervous System; Lymphoma; Waldenstrom Macroglobulinemia; Leukemia, Myeloid, Acute; Urologic Neoplasms; Amyloidosis; Lymphoma, Primary Effusion; Lupus Erythematosus, Systemic; Lymphoma, Extranodal NK-T-Cell; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Kidney Neoplasms; Urinary Bladder Neoplasms; Multiple Myeloma; Glioblastoma; Immunoglobulin Light-chain Amyloidosis; Myelodysplastic Syndromes; Lymphoma, Large B-Cell, Diffuse; Smoldering Multiple Myeloma; Autoimmune Diseases; Paraproteinemias; Genital Neoplasms, Male; Carcinoma, Renal Cell; Purpura, Thrombocytopenic, Idiopathic | Details |
| Daratumumab/Hyaluronidase-fihj | Approved | Johnson & Johnson Innovative Medicine | Darzalex Faspro, Darzquro | United States | Multiple Myeloma | Janssen Biotech Inc | 2020-05-01 | Peripheral Nervous System Diseases; Multiple Myeloma; Monoclonal Gammopathy of Undetermined Significance; Rejection of organ transplantation; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Amyloidosis; Rejection in heart transplantation | Details | |
| Isatuximab | SAR-650984 | Approved | Immunogen Inc | Sarclisa | United States | Multiple Myeloma | Sanofi-Aventis U.S. Llc | 2020-03-02 | Anemia, Aplastic; Leukemia, T-Cell; Carcinoma, Non-Small-Cell Lung; Macroglossia; Leukemia, Myeloid, Acute; Lymphoma, Non-Hodgkin; Lymphoma; Nausea; Hepatomegaly; Gastrointestinal Hemorrhage; Satiety Response; Diarrhea; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Hematologic Neoplasms; Anemia, Hemolytic, Autoimmune; Prostatic Neoplasms; Multiple Myeloma; Immunoglobulin Light-chain Amyloidosis; Purpura; Neoplasms; Smoldering Multiple Myeloma; Hodgkin Disease; Lymphadenopathy; Paraproteinemias; Constipation; Goiter, Nodular | Details |
| Daratumumab | JNJ-54767414 | Approved | Johnson & Johnson Innovative Medicine | Darzalex, 兆珂速, 兆珂 | United States | Multiple Myeloma | Janssen Biotech Inc | 2015-11-16 | Prostatic Neoplasms; POEMS Syndrome; Autoimmune Diseases of the Nervous System; Lymphoma; Waldenstrom Macroglobulinemia; Leukemia, Myeloid, Acute; Urologic Neoplasms; Amyloidosis; Lymphoma, Primary Effusion; Lupus Erythematosus, Systemic; Lymphoma, Extranodal NK-T-Cell; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Kidney Neoplasms; Urinary Bladder Neoplasms; Multiple Myeloma; Glioblastoma; Immunoglobulin Light-chain Amyloidosis; Myelodysplastic Syndromes; Lymphoma, Large B-Cell, Diffuse; Smoldering Multiple Myeloma; Autoimmune Diseases; Paraproteinemias; Genital Neoplasms, Male; Carcinoma, Renal Cell; Purpura, Thrombocytopenic, Idiopathic | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Felzartamab | MOR-03087; TJ-202; HBIB-202; MOR-202 | Phase 3 Clinical | Morphosys Ag | Glomerulonephritis, IGA; Lupus Nephritis; Multiple Myeloma; Glomerulonephritis, Membranous; Rejection of organ transplantation; Lupus Erythematosus, Systemic; Glomerulonephritis | Details |
| Daratumumab biosimilar (Biocad) | BCD-264 | Phase 3 Clinical | Biocad | Multiple Myeloma | Details |
| Mezagitamab | TAK-079 | Phase 2 Clinical | Takeda | Purpura, Thrombocytopenic, Idiopathic; Myasthenia Gravis; Hematologic Neoplasms; Nephrosis; Glomerulonephritis, IGA; Kidney Diseases; Autoimmune Diseases; Multiple Myeloma; Lupus Erythematosus, Systemic; Thrombocytopenia; Glomerulonephritis | Details |
| STI-6129 | STI-6129; LNDS-1001 | Phase 2 Clinical | Sorrento Therapeutics Inc | Solid tumours; Immunoglobulin Light-chain Amyloidosis; Multiple Myeloma; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute | Details |
| Modakafusp alfa | TEV-48573; TAK-573 | Phase 2 Clinical | Takeda Pharmaceutical Co Ltd | Neoplasms; Multiple Myeloma; Melanoma | Details |
| 4SCAR-T cell therapy (Shenzhen Geno-Immune Medical Institute) | 4SCAR-T | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Hematologic Neoplasms; Autoimmune Diseases; Neuroblastoma | Details |
| Anti-CD38 Antibody(Institute of Hematology & Blood Diseases Hospital) | Phase 2 Clinical | Institute Of Hematology & Blood Diseases Hospital | Purpura, Thrombocytopenic, Idiopathic; Antiphospholipid Syndrome | Details | |
| ISB-1442 | ISB 1442; ISB-1442 | Phase 2 Clinical | Ichnos Sciences Sa | Multiple Myeloma; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute | Details |
| Erzotabart | GEN-3014 | Phase 2 Clinical | Genmab A/S | Lymphoma, Large B-Cell, Diffuse; Leukemia, Myeloid, Acute | Details |
| BHV-1100 | KP-1237; BHV-1100 | Phase 2 Clinical | Biohaven Pharmaceuticals Inc, Kleo Pharmaceuticals, PeptiDream Inc | Multiple Myeloma | Details |
| Lumrotatug | CM-313 | Phase 2 Clinical | Keymed Biosciences Co Ltd | Hematologic Neoplasms; Multiple Myeloma; Lupus Erythematosus, Systemic; Lymphoma | Details |
| 89Zr-DFO-daratumumab | Phase 2 Clinical | Memorial Sloan Kettering Cancer Center | Multiple Myeloma | Details | |
| CART-38 | CART-38 | Phase 2 Clinical | The First Affiliated Hospital Of Soochow University | Leukemia, Myeloid, Acute | Details |
| 64Cu-DOTA-daratumumab | Phase 1 Clinical | City Of Hope National Medical Center | Multiple Myeloma | Details | |
| SAR-442257 | SAR-442257 | Phase 1 Clinical | Sanofi | Neoplasms | Details |
| Anti-BCMA anti-CD38 bispecific chimeric antigen receptor T cell therapy (Shengyan Pharmaceutical Technology) | Phase 1 Clinical | Shengyan Pharmaceutical Technology, Wuhan Union Hospital | Multiple Myeloma | Details | |
| Anti-CD38 chimeric antigen receptor T cell therapy(Yake Biotechnology) | CAR-T CD-38 | Phase 1 Clinical | Shanghai YaKe Biotechnology Co Ltd | Multiple Myeloma; Leukemia, Myeloid, Acute | Details |
| SCTC-21-C | SCTC21C; SCTC-21-C; SCT-C21C | Phase 1 Clinical | SinoCelltech Ltd | Hematologic Neoplasms | Details |
| CLL1 and CD38 Dual CAR-T therapy(Gracell Biotechnologies) | Phase 1 Clinical | Gracell Biotechnologies (Shanghai) Co Ltd, The 920th Hospital Of Joint Logistics Support Force Of PLA | Leukemia, Myeloid, Acute | Details | |
| Universal CAR-T Cells therapy(Shenzhen Geno-Immune Medical Institute) | Phase 1 Clinical | Shenzhen Geno-Immune Medical Institute | Leukemia, Myeloid, Acute | Details | |
| CD38-SADA:177 Lu-DOTA Drug Complex | Phase 1 Clinical | Y-Mabs Therapeutics Inc | Lymphoma, Non-Hodgkin | Details | |
| Daratumumab biosimilar (Hangzhou Jiuyuan Gene Engineering) | Phase 1 Clinical | Hangzhou Jiuyuan Gene Engineering Co Ltd | Multiple Myeloma | Details | |
| CART-38(University of Pennsylvania) | CART-38 | Phase 1 Clinical | University Of Pennsylvania | Multiple Myeloma; Leukemia, Myeloid, Acute | Details |
| ISB-2001 | ISB-2001 | Phase 1 Clinical | Ichnos Sciences Sa | Multiple Myeloma | Details |
| TNB-738 | TNB-738 | Phase 1 Clinical | TeneoFour Inc | Details | |
| GBR-1342 | GBR-1342; ISB-1342 | Phase 1 Clinical | Glenmark Pharmaceuticals Ltd | Multiple Myeloma | Details |
| XmAb-968 | XmAb968 | Phase 1 Clinical | Xencor Inc | Hematologic Neoplasms; Leukemia, Promyelocytic, Acute | Details |
| SG-2501 | SG-2501 | Phase 1 Clinical | Hangzhou Sumgen Biotechnology Co Ltd | Hematologic Neoplasms; Lymphoma | Details |
| XmAb-18968 | XmAb-18968 | Phase 1 Clinical | The Medical College Of Wisconsin Nonprofit | Leukemia, Myeloid, Acute | Details |
| CID-103 | CID-103; TSK011010 | Phase 1 Clinical | Tusk Therapeutics Ltd | Multiple Myeloma | Details |
| Daratumumab biosimilar (Henlius) | HLX-15 | Phase 1 Clinical | Shanghai Henlius Biologics Co Ltd | Multiple Myeloma | Details |
| 211At-OKT10-B10 | Phase 1 Clinical | Fred Hutchinson Cancer Research Center | Multiple Myeloma | Details | |
| Y-150(Wuhan Yzy Biopharma/CSPC Pharmaceutical) | Y-150; Y150 | Phase 1 Clinical | Wuhan Yzy Biopharma Co Ltd, CSPC Pharmaceutical Group Ltd | Multiple Myeloma | Details |
| Recombinant human anti-CD38 momoclonal antibody(Sumgen) | SG-301; SG301 | Phase 1 Clinical | Hangzhou Sumgen Biotechnology Co Ltd | Hematologic Neoplasms; Multiple Myeloma; Lupus Erythematosus, Systemic | Details |
| Anti CD38 chimeric antigen receptor T-cell therapy (Sorrento Therapeutics) | Phase 1 Clinical | Celularity Inc, Sorrento Therapeutics Inc | Multiple Myeloma | Details | |
| Felzartamab | MOR-03087; TJ-202; HBIB-202; MOR-202 | Phase 3 Clinical | Morphosys Ag | Glomerulonephritis, IGA; Lupus Nephritis; Multiple Myeloma; Glomerulonephritis, Membranous; Rejection of organ transplantation; Lupus Erythematosus, Systemic; Glomerulonephritis | Details |
| Daratumumab biosimilar (Biocad) | BCD-264 | Phase 3 Clinical | Biocad | Multiple Myeloma | Details |
| Mezagitamab | TAK-079 | Phase 2 Clinical | Takeda | Purpura, Thrombocytopenic, Idiopathic; Myasthenia Gravis; Hematologic Neoplasms; Nephrosis; Glomerulonephritis, IGA; Kidney Diseases; Autoimmune Diseases; Multiple Myeloma; Lupus Erythematosus, Systemic; Thrombocytopenia; Glomerulonephritis | Details |
| STI-6129 | STI-6129; LNDS-1001 | Phase 2 Clinical | Sorrento Therapeutics Inc | Solid tumours; Immunoglobulin Light-chain Amyloidosis; Multiple Myeloma; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute | Details |
| Modakafusp alfa | TEV-48573; TAK-573 | Phase 2 Clinical | Takeda Pharmaceutical Co Ltd | Neoplasms; Multiple Myeloma; Melanoma | Details |
| 4SCAR-T cell therapy (Shenzhen Geno-Immune Medical Institute) | 4SCAR-T | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Hematologic Neoplasms; Autoimmune Diseases; Neuroblastoma | Details |
| Anti-CD38 Antibody(Institute of Hematology & Blood Diseases Hospital) | Phase 2 Clinical | Institute Of Hematology & Blood Diseases Hospital | Purpura, Thrombocytopenic, Idiopathic; Antiphospholipid Syndrome | Details | |
| ISB-1442 | ISB 1442; ISB-1442 | Phase 2 Clinical | Ichnos Sciences Sa | Multiple Myeloma; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute | Details |
| Erzotabart | GEN-3014 | Phase 2 Clinical | Genmab A/S | Lymphoma, Large B-Cell, Diffuse; Leukemia, Myeloid, Acute | Details |
| BHV-1100 | KP-1237; BHV-1100 | Phase 2 Clinical | Biohaven Pharmaceuticals Inc, Kleo Pharmaceuticals, PeptiDream Inc | Multiple Myeloma | Details |
| Lumrotatug | CM-313 | Phase 2 Clinical | Keymed Biosciences Co Ltd | Hematologic Neoplasms; Multiple Myeloma; Lupus Erythematosus, Systemic; Lymphoma | Details |
| 89Zr-DFO-daratumumab | Phase 2 Clinical | Memorial Sloan Kettering Cancer Center | Multiple Myeloma | Details | |
| CART-38 | CART-38 | Phase 2 Clinical | The First Affiliated Hospital Of Soochow University | Leukemia, Myeloid, Acute | Details |
| 64Cu-DOTA-daratumumab | Phase 1 Clinical | City Of Hope National Medical Center | Multiple Myeloma | Details | |
| SAR-442257 | SAR-442257 | Phase 1 Clinical | Sanofi | Neoplasms | Details |
| Anti-BCMA anti-CD38 bispecific chimeric antigen receptor T cell therapy (Shengyan Pharmaceutical Technology) | Phase 1 Clinical | Shengyan Pharmaceutical Technology, Wuhan Union Hospital | Multiple Myeloma | Details | |
| Anti-CD38 chimeric antigen receptor T cell therapy(Yake Biotechnology) | CAR-T CD-38 | Phase 1 Clinical | Shanghai YaKe Biotechnology Co Ltd | Multiple Myeloma; Leukemia, Myeloid, Acute | Details |
| SCTC-21-C | SCTC21C; SCTC-21-C; SCT-C21C | Phase 1 Clinical | SinoCelltech Ltd | Hematologic Neoplasms | Details |
| CLL1 and CD38 Dual CAR-T therapy(Gracell Biotechnologies) | Phase 1 Clinical | Gracell Biotechnologies (Shanghai) Co Ltd, The 920th Hospital Of Joint Logistics Support Force Of PLA | Leukemia, Myeloid, Acute | Details | |
| Universal CAR-T Cells therapy(Shenzhen Geno-Immune Medical Institute) | Phase 1 Clinical | Shenzhen Geno-Immune Medical Institute | Leukemia, Myeloid, Acute | Details | |
| CD38-SADA:177 Lu-DOTA Drug Complex | Phase 1 Clinical | Y-Mabs Therapeutics Inc | Lymphoma, Non-Hodgkin | Details | |
| Daratumumab biosimilar (Hangzhou Jiuyuan Gene Engineering) | Phase 1 Clinical | Hangzhou Jiuyuan Gene Engineering Co Ltd | Multiple Myeloma | Details | |
| CART-38(University of Pennsylvania) | CART-38 | Phase 1 Clinical | University Of Pennsylvania | Multiple Myeloma; Leukemia, Myeloid, Acute | Details |
| ISB-2001 | ISB-2001 | Phase 1 Clinical | Ichnos Sciences Sa | Multiple Myeloma | Details |
| TNB-738 | TNB-738 | Phase 1 Clinical | TeneoFour Inc | Details | |
| GBR-1342 | GBR-1342; ISB-1342 | Phase 1 Clinical | Glenmark Pharmaceuticals Ltd | Multiple Myeloma | Details |
| XmAb-968 | XmAb968 | Phase 1 Clinical | Xencor Inc | Hematologic Neoplasms; Leukemia, Promyelocytic, Acute | Details |
| SG-2501 | SG-2501 | Phase 1 Clinical | Hangzhou Sumgen Biotechnology Co Ltd | Hematologic Neoplasms; Lymphoma | Details |
| XmAb-18968 | XmAb-18968 | Phase 1 Clinical | The Medical College Of Wisconsin Nonprofit | Leukemia, Myeloid, Acute | Details |
| CID-103 | CID-103; TSK011010 | Phase 1 Clinical | Tusk Therapeutics Ltd | Multiple Myeloma | Details |
| Daratumumab biosimilar (Henlius) | HLX-15 | Phase 1 Clinical | Shanghai Henlius Biologics Co Ltd | Multiple Myeloma | Details |
| 211At-OKT10-B10 | Phase 1 Clinical | Fred Hutchinson Cancer Research Center | Multiple Myeloma | Details | |
| Y-150(Wuhan Yzy Biopharma/CSPC Pharmaceutical) | Y-150; Y150 | Phase 1 Clinical | Wuhan Yzy Biopharma Co Ltd, CSPC Pharmaceutical Group Ltd | Multiple Myeloma | Details |
| Recombinant human anti-CD38 momoclonal antibody(Sumgen) | SG-301; SG301 | Phase 1 Clinical | Hangzhou Sumgen Biotechnology Co Ltd | Hematologic Neoplasms; Multiple Myeloma; Lupus Erythematosus, Systemic | Details |
| Anti CD38 chimeric antigen receptor T-cell therapy (Sorrento Therapeutics) | Phase 1 Clinical | Celularity Inc, Sorrento Therapeutics Inc | Multiple Myeloma | Details |
This web search service is supported by Google Inc.
