
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions

This protein carries a polyhistidine tag at the C-terminus.
The protein has a calculated MW of 70.5 kDa. The protein migrates as 95-110 kDa when calibrated against Star Ribbon Pre-stained Protein Marker under reducing (R) condition (SDS-PAGE) due to glycosylation.
>95% as determined by SDS-PAGE.
>90% as determined by SEC-MALS.
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
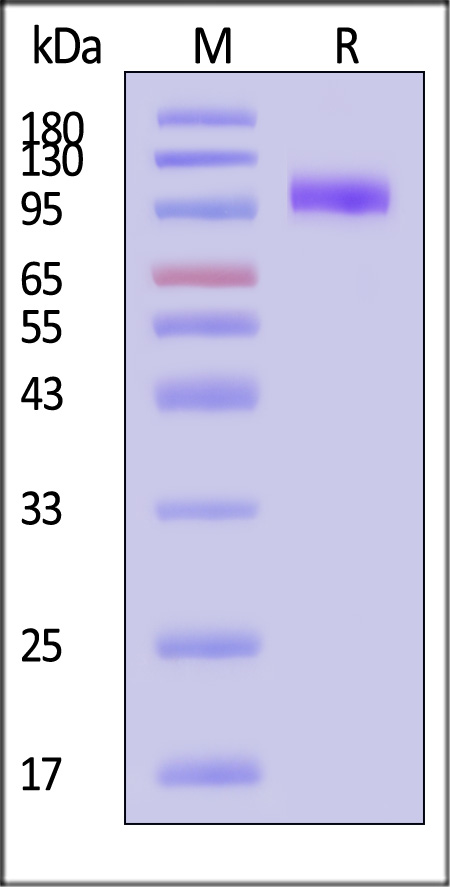
Human EGF R, His Tag on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95% (With Star Ribbon Pre-stained Protein Marker).
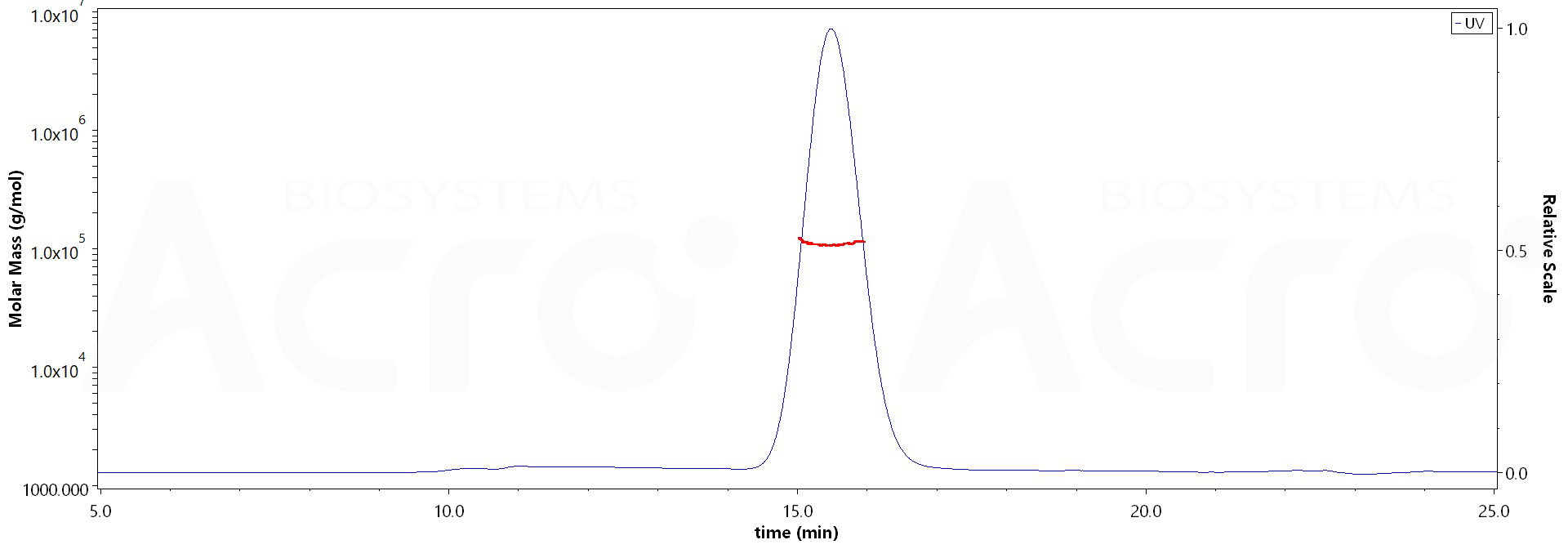
The purity of Human EGF R, His Tag (Cat. No. EGR-H5222) is more than 90% and the molecular weight of this protein is around 80-105 kDa verified by SEC-MALS.
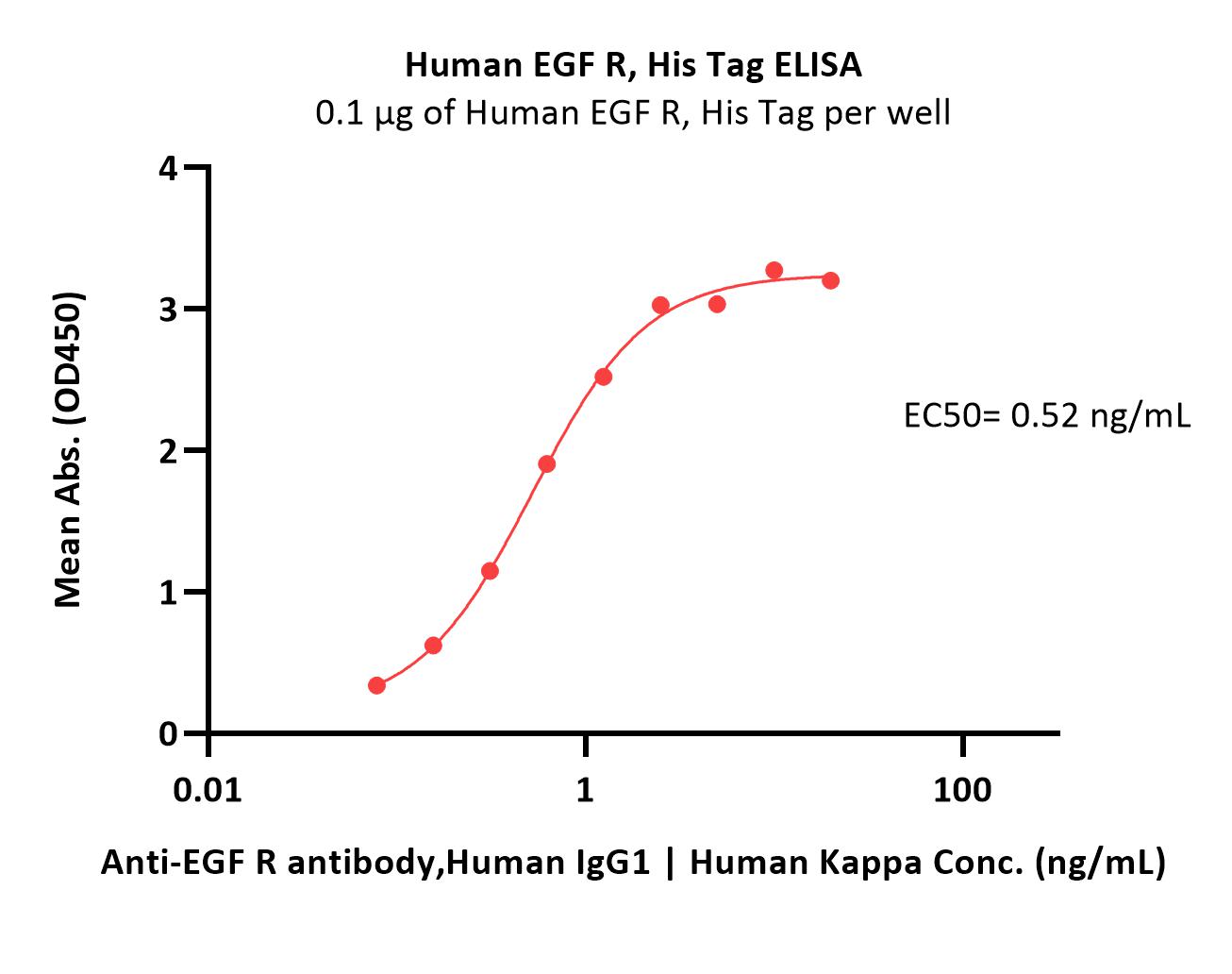
Immobilized Human EGF R, His Tag (Cat. No. EGR-H5222) at 1 μg/mL (100 μL/well) can bind Anti-EGF R antibody,Human IgG1 | Human Kappa with a linear range of 0.12-1.25 ng/mL (QC tested).
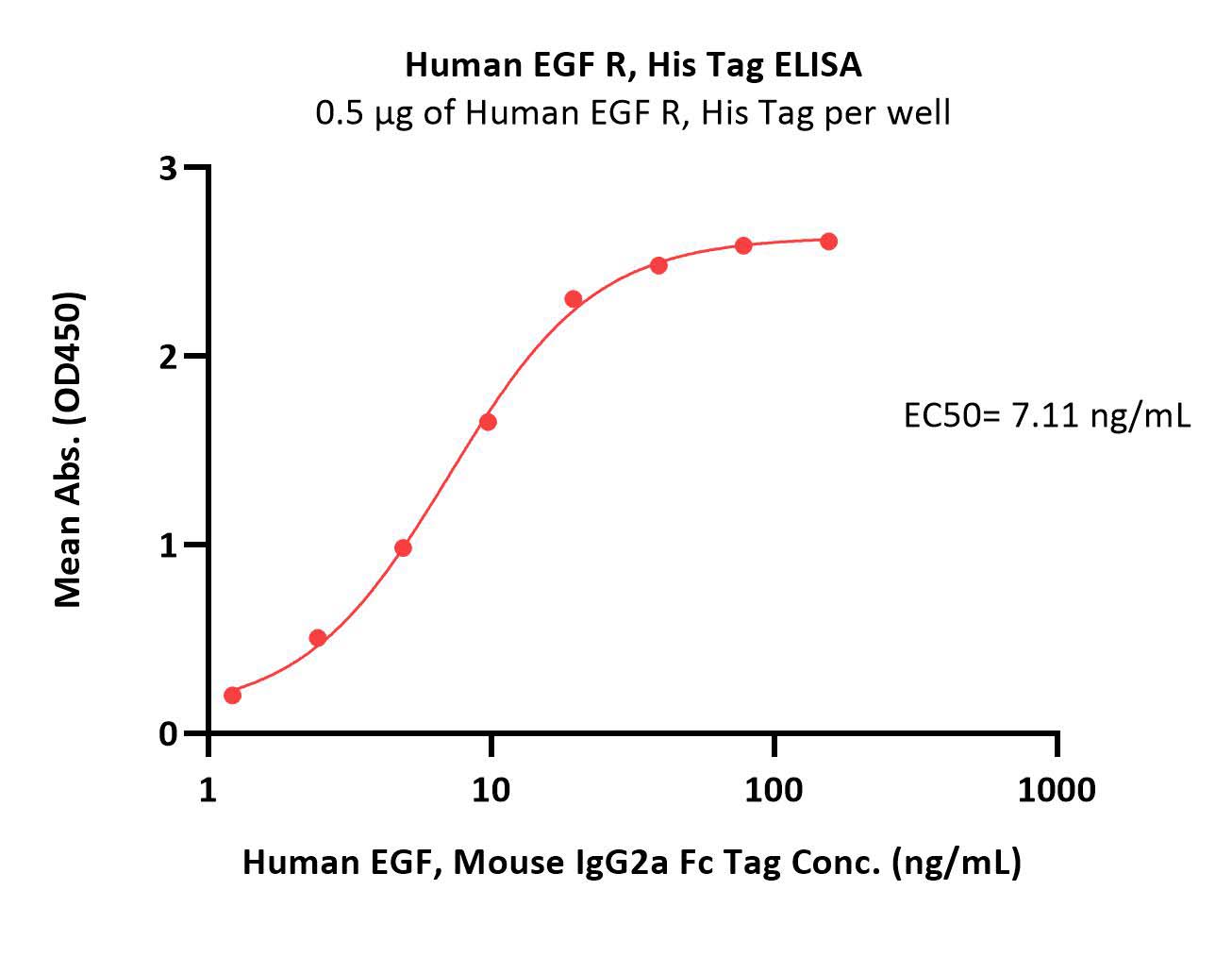
Immobilized Human EGF R, His Tag (Cat. No. EGR-H5222) at 5 μg/mL (100 μL/well) can bind Human EGF, Mouse IgG2a Fc Tag (Cat. No. EGF-H525b) with a linear range of 1-20 ng/mL (Routinely tested).
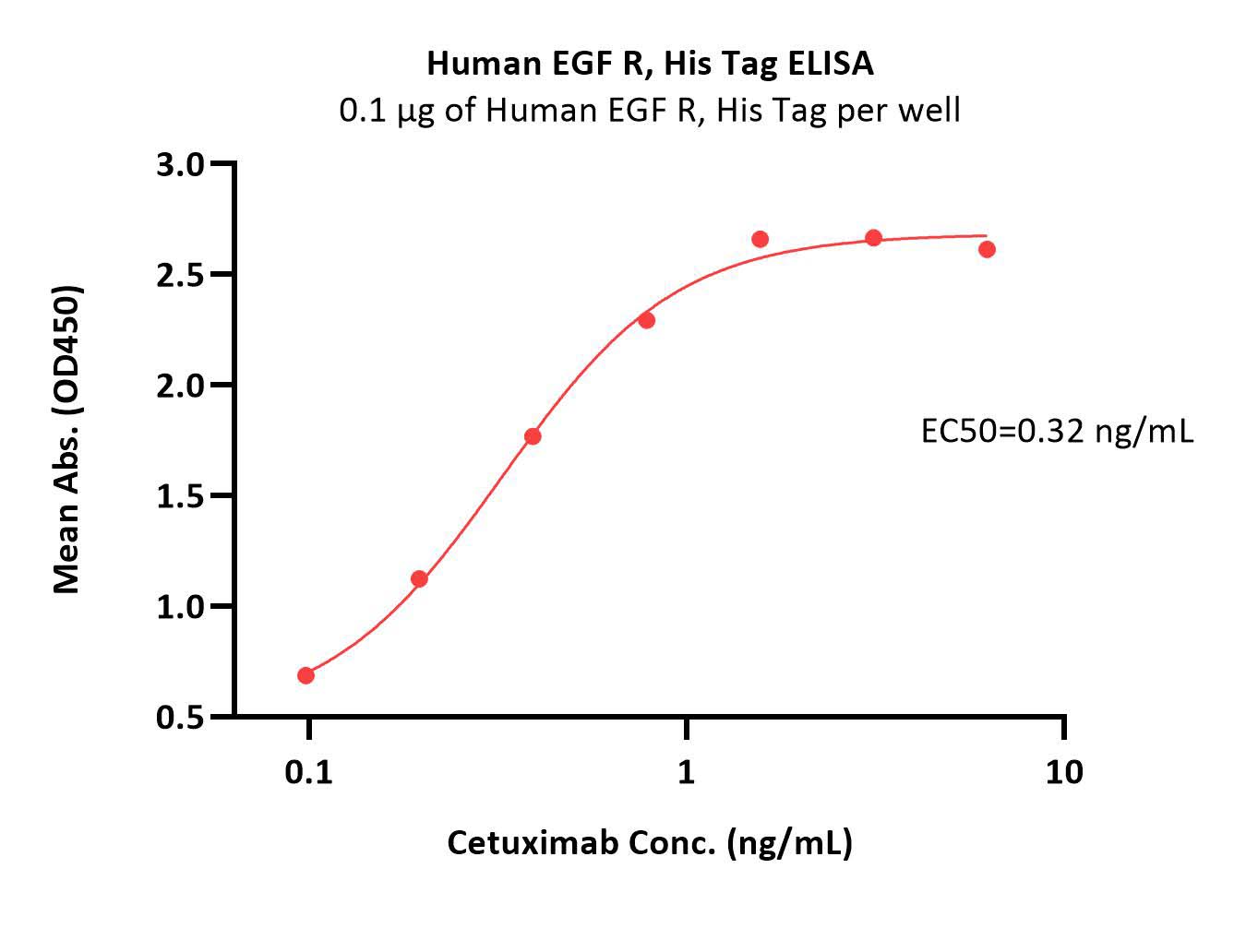
Immobilized Human EGF R, His Tag (Cat. No. EGR-H5222) at 1 μg/mL (100 μL/well) can bind Cetuximab with a linear range of 0.1-1 ng/mL (Routinely tested).
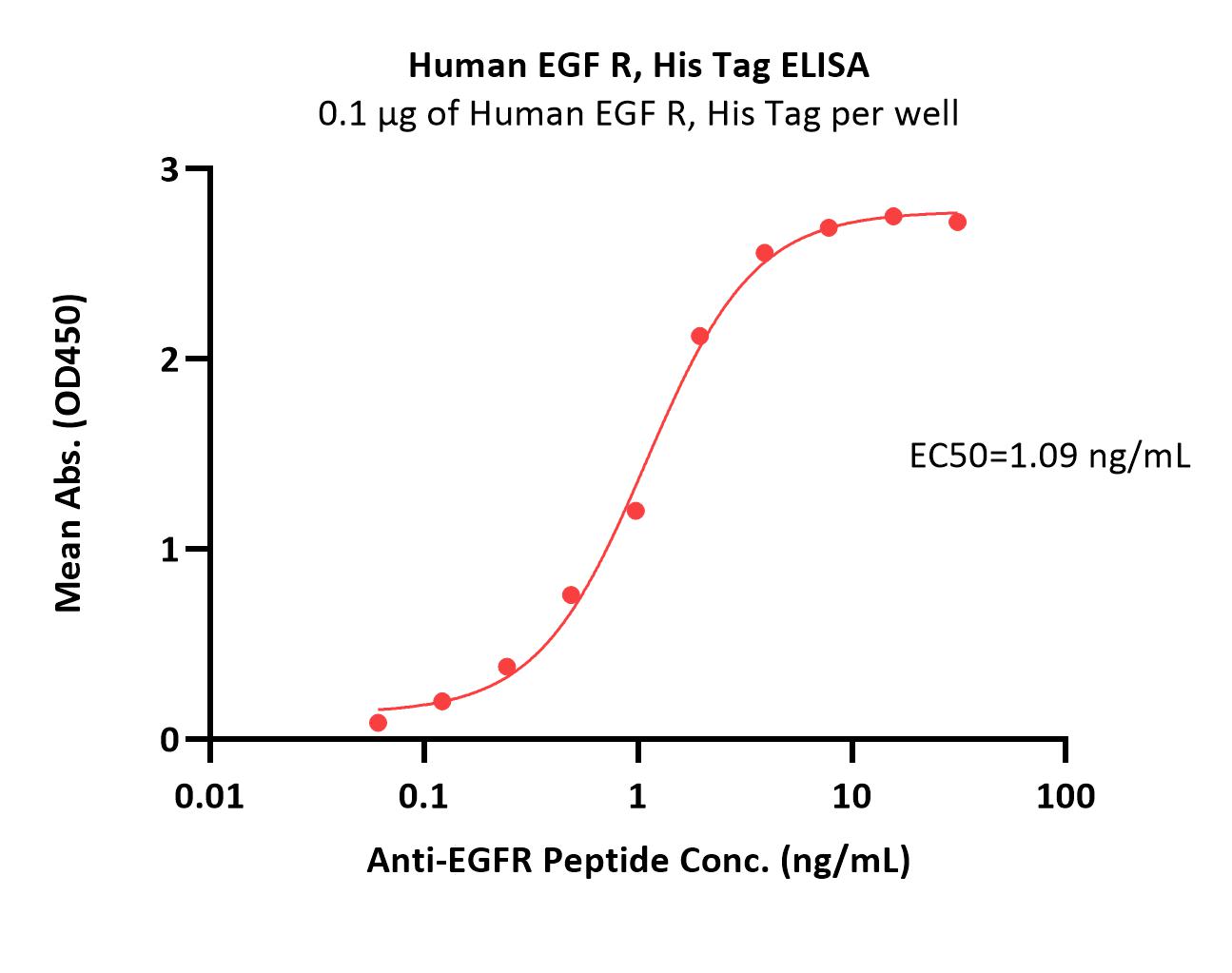
Immobilized Human EGF R, His Tag (Cat. No. EGR-H5222) at 1 μg/mL (100 μL/well) can bind Anti-EGFR Peptide with a linear range of 0.06-2 ng/mL (Routinely tested).
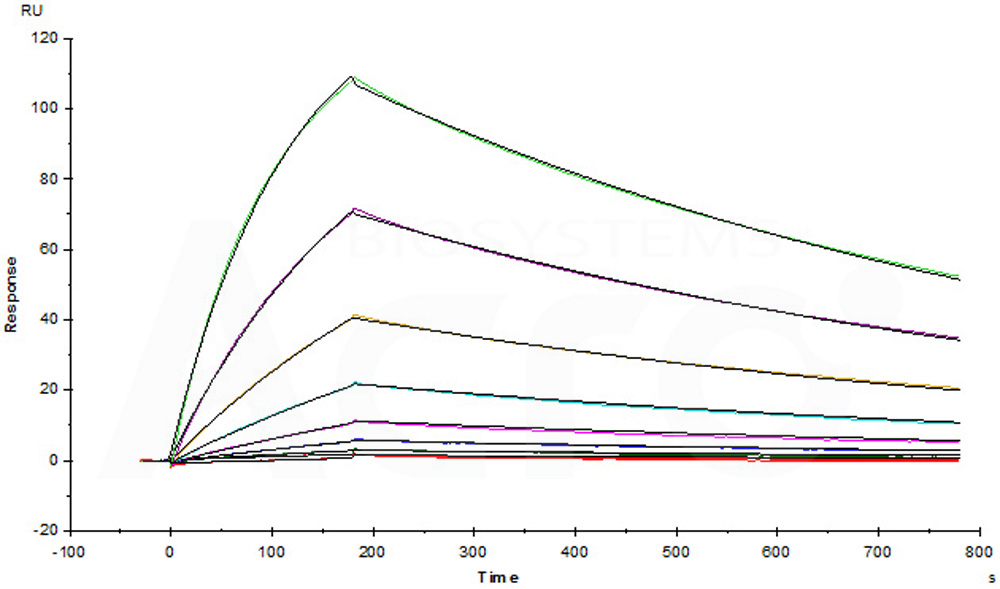
Erbitux (Cetuximab) captured on CM5 chip via anti-human IgG Fc antibodies surface, can bind Human EGF R, His Tag (Cat. No. EGR-H5222) with an affinity constant of 1.3 nM as determined in a SPR assay (Biacore T200) (Routinely tested).
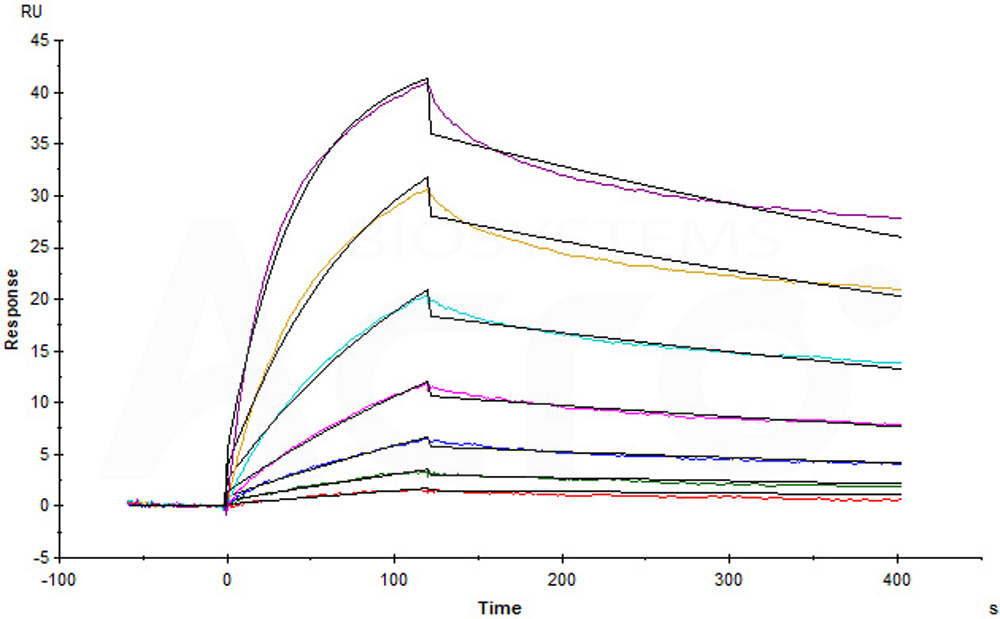
Human EGF R, His Tag (Cat. No. EGR-H5222) captured on CM5 Chip via anti-His antibody can bind Human EGF, Mouse IgG2a Fc Tag (Cat. No. EGF-H525b) with an affinity constant of 2.63 nM as determined in SPR assay (Biacore T200) (Routinely tested).
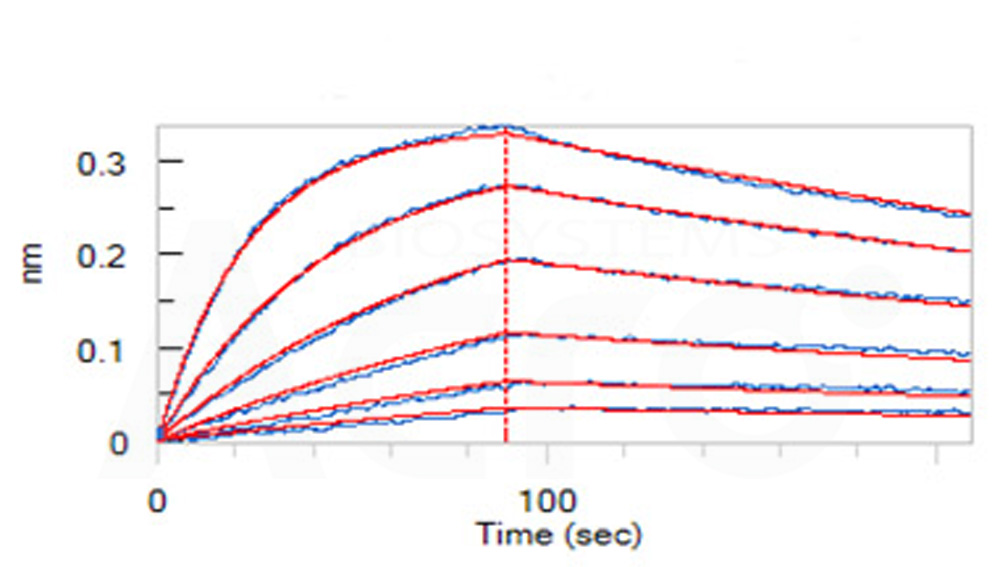
Loaded Erbitux (Cetuximab) on AHC Biosensor, can bind Human EGF R, His Tag (Cat. No. EGR-H5222) with an affinity constant of 1.23 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
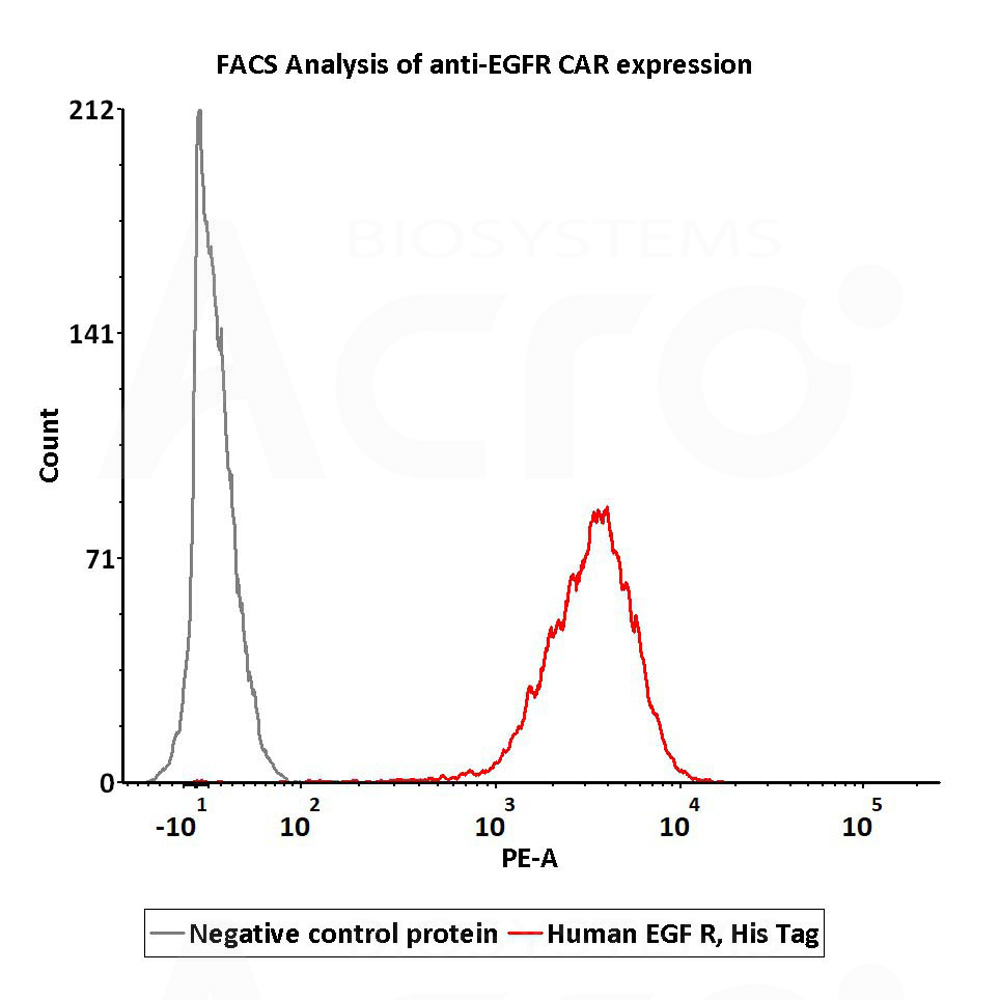
2e5 of Anti-EGFR CAR-293 cells were stained with 100 μL of 1 μg/mL of Human EGF R, His Tag (Cat. No. EGR-H5222) and negative control protein respectively, washed and then followed by PE anti-His antibody and analyzed with FACS (Routinely tested).

Explore our catalog of therapeutic antibody solutions to find the right products for you! We are dedicated to delivering solutions designed to help you drive innovation and push the boundaries of what therapeutic antibodies can be.

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

Organoid Toolbox is a collection of organoid solutions including ready-to-use organoids, organoid differentiation kits, and a variety of services to accelerate the progress of your drug development project.

ACROBiosystems developed a series of GMP grade cytokines under the GMP grade quality management system. Those products are all suitable for T/NK cell generation, activation, and proliferation in cell therapy research.

50+ targets designed for CAR detection, including PE/FITC/biotin labeled proteins. The key reagents for CD19 and BCMA were FDA DMF filed which can support your IND, NDA and BLA process.

Full length multi-pass TPs with stabilized structure and high bioactivity for immunization, antibody screening, cell based assay and CAR detection, including hot CD20, Claudin 18.2, CD133, GPRC5D,CCR8, CCR5, etc.

GMP grade cytokines, reagents for cell activation, gene edition, DNA/RNA removal, etc. Particularly focus on product design, quality control and solution-based support to link each phase of your cell and gene therapy journey.

A series of immune checkpoints including classic co-inhibitory and co-stimulatory receptors. The comprehensive catalog contains 100+ targets with various species and tags, and the high-quality proteins are in good batch-to-batch consistency.

To meet the needs of ADCs development, ACROBiosystems can provide: A variety of high-quality target proteins; MMPs/Cathepsin/uPA for cleavable linker; Anti-payload antibodies & anti-idiotypic antibodies for immunogenicity and PK analysis; SPR/BLI analytical and ADA development service.

Comprehensive collection of Fc receptor proteins, including their common variants, which can help expedite your antibody development.

Comprehensive cytokine targets including interleukins, growth factors, chemokines, TNFs, etc. are expressed by HEK293 to ensure their natural structure. Their high purity is verified by SDS-PAGE/HPLC/SEC-MALS and high bioactivity is verified by ELISA/SPR/BLI.

Aneuro provides innovative solutions for neuroscience research. Recombinant proteins, neural factors, pre-formed fibrils, electrophysiological electrodes, as well as Organoid Toolbox all in Aneuro aiming to advance neuroscience research, develop therapeutic interventions, and improve diagnostic methods for neurological diseases.
| English Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Osimertinib Mesylate | AZD-9291; RDL94R2A16; AZD-9291 Mesylate; AZD9291 | Approved | Astrazeneca Plc | 泰瑞沙, Tagrisso | United States | Carcinoma, Non-Small-Cell Lung | Astrazeneca Pharmaceutical Co Ltd | 2015-11-13 | Uterine Neoplasms; Urinary Bladder Neoplasms; Multiple Myeloma; Prostatic Neoplasms; Cholangiocarcinoma; Breast Neoplasms; Meningeal Neoplasms; Colorectal Neoplasms; Lung Neoplasms; Brain metastases; Adenocarcinoma of Lung; Endometrial Neoplasms; Thyroid Neoplasms; Carcinoma, Squamous Cell; Lymphoma; Glioma; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Adenocarcinoma; Melanoma; Esophageal Neoplasms; Liver Neoplasms; Head and Neck Neoplasms; Neoplasm, Residual; Ovarian Neoplasms; Hematologic Neoplasms; Carcinoma, Renal Cell; Stomach Neoplasms; Rectal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Solid tumours; Neoplasms; Glioblastoma; Colonic Neoplasms; Carcinoma, Transitional Cell; Carcinoma, Ovarian Epithelial; Skin Neoplasms; Small Cell Lung Carcinoma; Pancreatic Neoplasms | Details |
| PX-070101 | PX-070101 | Approved | Praxis Pharmaceuticals | Colombia | Diabetic Foot | Praxis Pharmaceuticals | 2015-07-01 | Diabetic Foot | Details | |
| Cetuximab sarotalocan | RM-1929; ASP-1929 | Approved | Aspyrian Therapeutics | Akalux | Japan | Head and Neck Neoplasms | Rakuten Medical Inc | 2020-09-25 | Head and Neck Neoplasms; Squamous Cell Carcinoma of Head and Neck | Details |
| Cetuximab | IMC-C255; BMS-564717; EMD-271786; C-255; GT-MAB-5.2; ch-225; LY-2939777; NSC-714692 | Approved | Bristol-Myers Squibb Company, Eli Lilly And Company, Merck Serono | 爱必妥, Erbitux | United States | Colorectal Neoplasms | Imclone Gmbh | 2004-02-12 | Carcinoma, Squamous Cell; Oropharyngeal Neoplasms; Neuralgia; Colorectal Neoplasms; Peritoneal Neoplasms; Glioma; Lung Neoplasms; Endometrial Neoplasms; Esophageal Squamous Cell Carcinoma; Laryngeal Neoplasms; Nasopharyngeal Carcinoma; Adenocarcinoma, Mucinous; Fallopian Tube Neoplasms; Complex Regional Pain Syndromes; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Neoplasm Metastasis; Gastrointestinal Neoplasms; Precancerous Conditions; Glioblastoma; Liver Neoplasms; Head and Neck Neoplasms; Solid tumours; Ovarian Neoplasms; Pain; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Esophageal Neoplasms; Pancreatic Neoplasms; Neoplastic Cells, Circulating; Neoplasms; Small Cell Lung Carcinoma; Hypopharyngeal Neoplasms; Neoplasms, Squamous Cell; Colonic Neoplasms; Chordoma; Carcinoma, Adenoid Cystic; Sarcoma | Details |
| Afatinib Dimaleate | BIBW-2992-MA2; BIBW-2992 | Approved | C.H. Boehringer Sohn Ag & Co. Kg | Gilotrif, Giotrif, Tovok, 吉泰瑞, Tomtovok | United States | Carcinoma, Non-Small-Cell Lung | C.H. Boehringer Sohn Ag & Co. Kg | 2013-07-12 | Esophageal Squamous Cell Carcinoma; Urethral Neoplasms; Brain Neoplasms; Prostatic Neoplasms; Ureteral Neoplasms; Urologic Neoplasms; Colorectal Neoplasms; Lung Neoplasms; Carcinoma, Squamous Cell; Breast Neoplasms; Gallbladder Neoplasms; Uterine Neoplasms; Lymphoma; Glioma; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Neoplasm Metastasis; Small Cell Lung Carcinoma; Head and Neck Neoplasms; Solid tumours; Hematologic Neoplasms; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Stomach Neoplasms; Renal Insufficiency; Neoplasms; Rhabdomyosarcoma; Neuroectodermal Tumors; Glioblastoma; Neoplasms, Squamous Cell; Urinary Bladder Neoplasms; Chordoma; Liver Diseases; Multiple Myeloma | Details |
| Epidermal growth factor biosimilar (RAS Lifesciences) | Approved | Ras Lifesciences | India | Ras Lifesciences | 2012-10-01 | Details | ||||
| Recombinant epidermal growth factor gel (Pavay Gene Pharmaceutical) | Approved | Guilin Huanuowei Gene Pharmaceutical Co Ltd | 易孚 | Mainland China | Skin Ulcer; Burns | Guilin Huanuowei Gene Pharmaceutical Co Ltd | 2002-12-23 | Skin Ulcer; Burns | Details | |
| Nimotuzumab | OSAG-10; KI-0502; KI-0501; DE-766; h-R3; OSAG-101; TheraCIMh-R3; YMB-1000 | Approved | Center Of Molecular Immunology, Cimym Biosciences | BIOMAb-EGFR, TheraCIM, 泰欣生, CIMAher, Theraloc | India | Head and Neck Neoplasms; Nasopharyngeal Neoplasms | null | 2006-07-21 | Pancreatic neuroendocrine tumors (pNET); Esophageal Diseases; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Squamous Cell; Glioma; Esophageal Squamous Cell Carcinoma; Colorectal Neoplasms; Nasopharyngeal Carcinoma; Pancreatic Neoplasms; Head and Neck Neoplasms; Nasopharyngeal Diseases; Neoplasms; Nasopharyngeal Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Anus Neoplasms; Solid tumours | Details |
| Recombinant epidermal growth factor (Center for Genetic Engineering and Biotechnology/Praxis Pharmaceuticals) | Approved | Praxis Pharmaceuticals, Center For Genetic Engineering And Biotechnology | Heberprot-P | Cuba | Diabetic Foot | null | 2007-09-01 | Diabetic Foot | Details | |
| Erlotinib Hydrochloride | CP-358774-01; RO-0508231; NSC-718781; CP-358774; RG-1415; OSI-774; R-1415 | Approved | Genentech Inc | Tarceva, 特罗凯 | EU | Pancreatic Neoplasms; Carcinoma, Non-Small-Cell Lung | Roche Registration Gmbh | 2004-11-18 | Head and Neck Neoplasms; Kidney Neoplasms; Cystadenocarcinoma; Solid tumours; Ovarian Neoplasms; Liver Neoplasms; HIV Infections; Adenomatous Polyposis Coli; Neoplasm Recurrence, Local; Ependymoma; Brenner Tumor; Rhabdomyosarcoma; Medulloblastoma; Leukemia, Promyelocytic, Acute; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Cystadenocarcinoma, Mucinous; Anus Neoplasms; Esophageal Neoplasms; Esthesioneuroblastoma, Olfactory; Granuloma, Lethal Midline; Carcinoma, Basal Cell; Rectal Neoplasms; Cystadenocarcinoma, Serous; Polycythemia Vera; Anaplasia; Stomach Neoplasms; Neoplasms; Carcinoma, Transitional Cell; Salivary Gland Neoplasms; Pancreatic Neoplasms; Skin Neoplasms; Small Cell Lung Carcinoma; Myelodysplastic Syndromes; Leukemia, Myelomonocytic, Acute; Colonic Neoplasms; Carcinoma, Ovarian Epithelial; Glioblastoma; Nasopharyngeal Neoplasms; Adenocarcinoma of Lung; Fibrosis; Carcinoma, Verrucous; Liver Cirrhosis; Leukemia, Myelomonocytic, Chronic; Mesothelioma; Urinary Bladder Neoplasms; P | Details |
| Gefitinib | ZD-1839 | Approved | Astrazeneca Plc | Iressa, 易瑞沙 | Japan | Carcinoma, Non-Small-Cell Lung | Astrazeneca Pharmaceutical Co Ltd | 2002-07-05 | Adenocarcinoma, Bronchiolo-Alveolar; Lip Neoplasms; Leukemia, Myeloid, Acute; Glioma; Carcinoma, Squamous Cell; Brain metastases; Lung Neoplasms; Carcinoma, Mucoepidermoid; Astrocytoma; Gliosarcoma; Paranasal Sinus Neoplasms; Colorectal Neoplasms; Gastrinoma; Carcinoma, Adenosquamous; Nasopharyngeal Carcinoma; Medullary thyroid cancer (MTC); Urethral Neoplasms; Brain Neoplasms; Breast Neoplasms; Neoplasms, Germ Cell and Embryonal; Uterine Cervical Neoplasms; Tongue Neoplasms; Thyroid Cancer, Papillary; Somatostatinoma; Carcinoma, Hepatocellular; Breast Neoplasms, Male; Gastrointestinal Neoplasms; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Prostatic Neoplasms; Adenocarcinoma; Endometrial Neoplasms; Fallopian Tube Neoplasms; Thyroid Neoplasms; Neoplasms, Neuroepithelial; Lymphoma; Mouth Neoplasms; Laryngeal Neoplasms; Carcinoma, Renal Cell; Carcinoid Tumor; Parathyroid Neoplasms; Esthesioneuroblastoma, Olfactory; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Basal Cell; Granuloma, Lethal Midlin | Details |
| Brigatinib | AP-26113 | Approved | Ariad Pharmaceuticals Inc | Alunbrig, ALUNBRIG | United States | Carcinoma, Non-Small-Cell Lung | Takeda Pharmaceuticals U.S.A. Inc | 2017-04-28 | Solid tumours; Ependymoma; Carcinoma; Neoplasms; Neurofibromatosis 2; Small Cell Lung Carcinoma; Myofibroma; Lymphoma, Large-Cell, Anaplastic; Brain metastases; Lung Neoplasms; Granuloma, Plasma Cell; Carcinoma, Non-Small-Cell Lung; Sarcoma, Kaposi; Neurilemmoma; Meningioma; Neuroma, Acoustic | Details |
| Recombinant Human Epidermal Growth Factor Derivative (Shenzhen Wastin Genetech) | rEGF | Approved | Shenzhen Watsin Genetech Ltd | 金因肽, GeneTime | Mainland China | Wounds and Injuries | Shenzhen Watsin Genetech Ltd | 2001-01-01 | Wounds and Injuries | Details |
| Nepidermin | DWP-401 | Approved | Daewoong Pharmaceutical Co Ltd | Easyef | South Korea | Alopecia; Diabetic Foot | Daewoong Pharmaceutical Co Ltd | 2001-01-01 | Diabetic Foot; Alopecia; Dry Eye Syndromes | Details |
| Icotinib Hydrochloride | BPI-2009H; BPI-2009C | Approved | Betta Pharmaceuticals Co Ltd | 凯美纳, Conmana | Mainland China | Carcinoma, Non-Small-Cell Lung | Betta Pharmaceuticals Co Ltd | 2011-06-07 | Nasopharyngeal Carcinoma; Neuroma, Acoustic; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Carcinoma, Squamous Cell; Lung Neoplasms; Brain metastases; Breast Neoplasms; Carcinoma, Adenosquamous; Solid tumours; Psoriasis; Adenocarcinoma of Lung; Neurofibromatosis 2; Small Cell Lung Carcinoma; Bronchial Neoplasms; Stomach Neoplasms; Esophageal Neoplasms | Details |
| Recombinant human EGF conjugated vaccine | Approved | Biotech Pharmaceuticals Co Ltd | Carcinoma, Non-Small-Cell Lung | Details | ||||||
| Recombinant epidermal growth factor (Bharat Biotech) | REGEN-D 60; REGEN-D 150 | Approved | Bharat Biotech International Ltd | India | Diabetic Foot; Burns | Bharat Biotech International Ltd | 2005-01-01 | Diabetic Foot; Burns | Details | |
| Lazertinib | YH-25448; JNJ-73841937; JNJ-1937; GNS-1480 | Approved | Oscotec Inc, Janssen Biotech Inc, Yuhan Corp | Leclaza | South Korea | Carcinoma, Non-Small-Cell Lung | Yuhan Corp | 2021-01-18 | Hepatic Insufficiency; Carcinoma, Non-Small-Cell Lung | Details |
| Recombinant epidermal growth factor biosimilar (Elea) | r-hu-EGF | Approved | Elea | Argentina | Diabetic Foot; Burns | Elea | 2015-01-01 | Diabetic Foot; Burns | Details | |
| Recombinant Human Epidermal Growth Factor Derivative Eye Drops (Shenzhen Wastin Genetech) | Approved | Shenzhen Watsin Genetech Ltd | 金因舒, GeneSoft | Mainland China | Corneal Diseases | Shenzhen Watsin Genetech Ltd | 2004-01-21 | Corneal Diseases | Details | |
| Necitumumab | LY-3012211; IMC-11F8 | Approved | Eli Lilly And Company | Portrazza | United States | Carcinoma, Non-Small-Cell Lung | Eli Lilly And Co (Ireland) Ltd | 2015-11-24 | Solid tumours; Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis | Details |
| Sunvozertinib | DZD-9008 | Approved | Dizal (Jiangsu) Pharmaceutical Co Ltd | 舒沃哲 | Mainland China | Carcinoma, Non-Small-Cell Lung | Dizal (Jiangsu) Pharmaceutical Co Ltd | 2023-08-22 | Lymphoma, B-Cell; Hepatic Insufficiency; Lymphoma, Non-Hodgkin; Carcinoma, Non-Small-Cell Lung | Details |
| Panitumumab | ABX-0303; E7.6.3; AMG-954; ABX-EGF; ABX-10221 | Approved | Amgen Inc | Vectibix | United States | Colorectal Neoplasms | Amgen Inc | 2006-09-27 | Small Cell Lung Carcinoma; Exanthema; Carcinoma, Non-Small-Cell Lung; Gastrointestinal Neoplasms; Neoplasm Metastasis; Glioma; Carcinoma, Squamous Cell; Lung Neoplasms; Adenoma, Pleomorphic; Colorectal Neoplasms; Brain Neoplasms; Prostatic Neoplasms; Colonic Neoplasms; Head and Neck Neoplasms; Pancreatic Neoplasms; Neoplasms; Malignant Carcinoid Syndrome; Salivary Gland Neoplasms; Rectal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Esophageal Neoplasms; Carcinoma; Kidney Neoplasms; Solid tumours | Details |
| Neratinib Maleate | CAN-030; PB-272; HKI-272; PF-0528767; WAY-179272 | Approved | Pfizer Inc | Nerlynx, 贺俪安 | United States | Breast Neoplasms | Puma Biotechnology Inc | 2017-07-17 | Solid tumours; Esophageal Neoplasms; Neoplasms; Breast Neoplasms; Hepatic Insufficiency; Colorectal Neoplasms; Lung Neoplasms; Brain metastases; Metastatic breast cancer; Carcinoma, Non-Small-Cell Lung | Details |
| Cetuximab biosimilar (CinnaGen) | Approved | Cinnagen | Iran | Colorectal Neoplasms | Cinnagen | 2017-01-01 | Colorectal Neoplasms | Details | ||
| Lapatinib Ditosylate Hydrate | GW-572016; GW-572016F; GW-2016 | Approved | Glaxosmithkline Plc, Novartis Pharma Ag | Tykerb, Tyverb, 泰立沙, Tykerb/Tyverb | United States | Breast Neoplasms | Novartis Pharma Ag | 2007-03-13 | Laryngeal Neoplasms; Prostatic Neoplasms; Gliosarcoma; Colorectal Neoplasms; Astrocytoma; Bile Duct Neoplasms; Carcinoma, Acinar Cell; Carcinoma, Mucoepidermoid; Endometrial Neoplasms; Neoplasms, Gonadal Tissue; Lung Neoplasms; Carcinoma, Squamous Cell; Glioma; Brain Neoplasms; Gallbladder Neoplasms; Brain metastases; Lymphoma; Uterine Cervical Neoplasms; Adenocarcinoma; Melanoma; Tongue Neoplasms; Breast Neoplasms, Male; Carcinoma, Non-Small-Cell Lung; Neuroma, Acoustic; Carcinoma, Hepatocellular; Neoplasm Metastasis; Glioblastoma; Ovarian Neoplasms; Ependymoma; Head and Neck Neoplasms; Liver Neoplasms; Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Carcinoma; Abdominal Neoplasms; Salivary Gland Neoplasms; Medulloblastoma; Carcinoma, Verrucous; Neoplasms; Neurofibromatosis 2; Small Cell Lung Carcinoma; Carcinoma, Ovarian Epithelial; Pancreatic Neoplasms; Central Nervous System Neoplasms; Urinary Bladder Neoplasms; Oligodendroglioma; Ca | Details |
| Recombinant epidermal growth factor (Shanghai Haohai ) | Approved | Shanghai Haohai Biological Technology Co Ltd | 康合素 | Mainland China | Burns | Shanghai Haohai Biological Technology Co Ltd | 2001-01-01 | Burns | Details | |
| Amivantamab | CNTO-4424; JNJ-61186372; CNTO4424; JNJ-372; JNJ-6372 | Approved | Genmab A/S, Johnson & Johnson Innovative Medicine | RYBREVANT | United States | Carcinoma, Non-Small-Cell Lung | Janssen Biotech Inc | 2021-05-21 | Esophageal Neoplasms; Stomach Neoplasms; Small Cell Lung Carcinoma; Carcinoma, Adenoid Cystic; Colorectal Neoplasms; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Carcinoma, Hepatocellular | Details |
| Recombinant epidermal growth factor drop (Pavay Gene Pharmaceutical) | Approved | Guilin Huanuowei Gene Pharmaceutical Co Ltd | 易贝 | Mainland China | Corneal Diseases | Guilin Huanuowei Gene Pharmaceutical Co Ltd | 2002-04-12 | Corneal Diseases; Xerophthalmia | Details | |
| Pyrotinib Maleate | HTI-1001; SHR-1258; BLTN | Approved | Jiangsu Hengrui Medicine Co Ltd | 艾瑞妮 | Mainland China | Breast Neoplasms | Jiangsu Hengrui Medicine Co Ltd | 2018-08-12 | Biliary Tract Neoplasms; Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Adenocarcinoma of Lung; Neoplasms; Breast Neoplasms; Bile Duct Neoplasms; Lung Neoplasms; Metastatic breast cancer; Gastrointestinal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Dacomitinib | PF-00299804-03; PF-00299804-3; PF-00299804; PF-299804; PF-299; PF-804 | Approved | Pfizer Inc | Vizimpro | United States | Carcinoma, Non-Small-Cell Lung | Pfizer Inc | 2018-09-27 | Brain Neoplasms; Adenocarcinoma; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Penile Neoplasms; Carcinoma, Squamous Cell; Esophageal Squamous Cell Carcinoma; Mouth Neoplasms; Lung Neoplasms; Colorectal Neoplasms; Solid tumours; Liver Diseases; Carcinoma, Large Cell; Glioblastoma; Neoplasms; Small Cell Lung Carcinoma; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Head and Neck Neoplasms | Details |
| Mouse epidermal growth factor (Hangzhou Tianmushan Pharmaceutical) | Approved | Hangzhou Tianmushan Pharmaceutical Enterprise Co Ltd | 一夫 | Wounds and Injuries | Details |
| English Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| PLB-1004 | PLB-1004 | Phase 3 Clinical | Beijing Avistone Pharmaceuticals Biotechnology Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| YK-029A | YK-029A; YK-209A; YK-209-A; YK-029-A | Phase 3 Clinical | Hainan Yuekang Biomedicine Co Ltd | Drug-Related Side Effects and Adverse Reactions; Carcinoma, Non-Small-Cell Lung | Details |
| Olafertinib | CK-101; RX-518; CS-2481; EGFR-IN-3 | Phase 3 Clinical | Suzhou Neupharma Co Ltd | Lung Diseases; Lung Neoplasms; Adenocarcinoma; Carcinoma, Non-Small-Cell Lung | Details |
| Mefatinib | Phase 3 Clinical | Suzhou Maitai Bio-Technology Co Ltd | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details | |
| Cetuximab biosimilar (AlphaMab) | KN-005 | Phase 3 Clinical | Suzhou Alphamab Co Ltd | Head and Neck Neoplasms; Anus Neoplasms; Urinary Bladder Neoplasms; Colorectal Neoplasms | Details |
| MRG-003 | MRG-003; MRG003 | Phase 3 Clinical | Head and Neck Neoplasms; Solid tumours; Biliary Tract Neoplasms; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Esophageal Neoplasms; Nasopharyngeal Carcinoma; Bile Duct Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| HA121-28 | HA121-28; SYHA121-28 | Phase 3 Clinical | Biliary Tract Neoplasms; Solid tumours; Esophageal Neoplasms; Neoplasms; Medullary thyroid cancer (MTC); Bile Duct Neoplasms; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| Nimotuzumab biosimilar (El Kendi Pharmaceuticals Manufacturing) | Phase 3 Clinical | El Kendi Pharmaceuticals | Uterine Cervical Neoplasms | Details | |
| Cetuximab biosimilar (AMPO Biotechnology) | Phase 3 Clinical | Ampo Biotechnology Inc | Colorectal Neoplasms | Details | |
| Cetuximab biosimilar (R-Pharm) | RPH-002 | Phase 3 Clinical | R-Pharm | Head and Neck Neoplasms; Pancreatic Neoplasms | Details |
| QL1203 | QL-1203 | Phase 3 Clinical | Qilu Pharmaceutical Co Ltd | Colorectal Neoplasms | Details |
| BEBT-109 | BEBT-109 | Phase 3 Clinical | Guangzhou BeBetter Medicine Technology Co | Carcinoma, Non-Small-Cell Lung | Details |
| Immunomodulatory progenitor cell therapy (Celixir) | Phase 3 Clinical | Celixir | Endomyocardial Fibrosis; Cardiomyopathies | Details | |
| Izalontamab | SI-B001; SI-1X6.4 | Phase 3 Clinical | Solid tumours; Head and Neck Neoplasms; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Neoplasms, Glandular and Epithelial; Triple Negative Breast Neoplasms; Digestive System Neoplasms; Colorectal Neoplasms; Carcinoma, Squamous Cell; Esophageal Squamous Cell Carcinoma; Carcinoma, Non-Small-Cell Lung | Details | |
| SKLB-1028 | SKLB-1028 | Phase 3 Clinical | Sichuan University, CSPC Pharmaceutical Group Ltd | Solid tumours; Leukemia, Promyelocytic, Acute; Leukemia, Myeloid; Leukemia, Myeloid, Acute | Details |
| Varlitinib Ditosylate | SPS-4370; ASLAN-001; ARRY-334543; ARRY-543; QBT-01 | Phase 3 Clinical | Array Biopharma | Biliary Tract Neoplasms; Solid tumours; Stomach Neoplasms; Neoplasms; Pancreatic Neoplasms; Breast Neoplasms; Cholangiocarcinoma; Bile Duct Neoplasms | Details |
| Becotatug | JMT-101 | Phase 3 Clinical | Shanghai Jmt-Bio Inc | Solid tumours; Rectal Neoplasms; Colorectal Neoplasms; Carcinoma, Squamous Cell; Esophageal Squamous Cell Carcinoma; Carcinoma, Non-Small-Cell Lung | Details |
| Izalontamab brengitecan | BL-B01D1 | Phase 3 Clinical | SystImmune | Nasopharyngeal Carcinoma; Gastrointestinal Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung; Metastatic breast cancer; Lung Neoplasms; Esophageal Squamous Cell Carcinoma; Colorectal Neoplasms; Urologic Neoplasms; Breast Neoplasms; Solid tumours; Digestive System Neoplasms; Small Cell Lung Carcinoma; Neoplasms; Carcinoma, Transitional Cell; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Esophageal Neoplasms; Neoplasms, Fibroepithelial | Details |
| Nimotuzumab biosimilar (IBC Generium) | Phase 3 Clinical | International Biotechnology Center Generium Llc | Head and Neck Neoplasms | Details | |
| Depatuxizumab mafodotin | ABT-414; ABT-414/806 | Phase 3 Clinical | Abbvie Inc | Glioblastoma; Gliosarcoma; Carcinoma, Squamous Cell; Glioma | Details |
| EG-007 | EG-007 | Phase 3 Clinical | Endometrial Neoplasms | Details | |
| Larotinib Mesylate | Z-650 | Phase 3 Clinical | Guangdong Dongyangguang Pharmaceutical Co Ltd | Solid tumours; Esophageal Neoplasms; Pancreatic Neoplasms; Esophageal Squamous Cell Carcinoma | Details |
| Ametumumab | SY-101 | Phase 2 Clinical | Institute of Bioengineering Academy of Military Medical Sciences Chinese People's Liberation Army, Shanghai Serum Bio-Technology Co Ltd | Solid tumours; Colorectal Neoplasms | Details |
| BB-401 | BB-401 | Phase 2 Clinical | Benitec Biopharma Ltd | Squamous Cell Carcinoma of Head and Neck | Details |
| Sutetinib Maleate | Phase 2 Clinical | Jiangsu Maidu Pharmaceutical R & D Co Ltd, Jiangsu Suzhong Pharma Group Co Ltd | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details | |
| Petosemtamab | MCLA-158 | Phase 2 Clinical | Merus Nv | Solid tumours; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Neoplasms; Digestive System Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Gastrointestinal Neoplasms; Neoplasm Metastasis | Details |
| GC-1118A | GC-1118; GC-1118A | Phase 2 Clinical | GC Biopharma Corp | Solid tumours; Stomach Neoplasms; Colorectal Neoplasms; Neoplasm Metastasis | Details |
| Durvalumab/Gefitinib | Phase 2 Clinical | Medimmune | Carcinoma, Non-Small-Cell Lung | Details | |
| Pamvatamig | MCLA-129 | Phase 2 Clinical | Merus Nv | Solid tumours; Head and Neck Neoplasms; Stomach Neoplasms; Squamous Cell Carcinoma of Head and Neck; Colorectal Neoplasms; Esophageal Squamous Cell Carcinoma; Carcinoma, Non-Small-Cell Lung | Details |
| JNJ-26483327 | JNJ-26483327; MTKi-327; BGB-102 | Phase 2 Clinical | Johnson & Johnson | Solid tumours; Neoplasms; Macular Degeneration | Details |
| HER-1 vaccine (Center of Molecular Immunology) | HER1-ECD; HER1-VSSP; HER-1-ECD-VSSP | Phase 2 Clinical | Center Of Molecular Immunology | Neoplasms | Details |
| AFM-24 | AFM-24 | Phase 2 Clinical | Affimed | Solid tumours | Details |
| C225-ILS-DOX | C225-ILs-dox | Phase 2 Clinical | Universitaetsspitals Basel | Breast Neoplasms | Details |
| Nazartinib | EGF-816; EGFRmut-TKI EGF816 | Phase 2 Clinical | Novartis Pharma Ag | Bronchial Neoplasms; Small Cell Lung Carcinoma; Triple Negative Breast Neoplasms; Colorectal Neoplasms; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma | Details |
| Sapitinib | AZD-8931 | Phase 2 Clinical | Astrazeneca Plc | Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis | Details |
| Tesevatinib | XL-647; KD-020; KD-019; EXEL-7647 | Phase 2 Clinical | Exelixis Inc | Glioblastoma; Neoplasms; Brain Neoplasms; Breast Neoplasms; Polycystic Kidney, Autosomal Dominant; Brain metastases; Polycystic Kidney, Autosomal Recessive; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung | Details |
| Futuximab/Modotuximab | Sym-004; S95026; S-95026; 992-and-1024 | Phase 2 Clinical | Symphogen A/S | Solid tumours; Carcinoma; Squamous Cell Carcinoma of Head and Neck; Colorectal Neoplasms; Glioma | Details |
| DBPR-112 | ABT-101; DBPR-112 | Phase 2 Clinical | National Health Research Institutes | Solid tumours; Head and Neck Neoplasms; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Panitumumab-IRDye800CW (Stanford University) | Phase 2 Clinical | Stanford University | Head and Neck Neoplasms; Squamous Cell Carcinoma of Head and Neck; Brain Neoplasms | Details | |
| AMX-3009 Maleate | AMX-3009; AMX3009马来酸 | Phase 2 Clinical | Anrun Medicine Technology (Suzhou) Co Ltd | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Pancreatic Neoplasms; Breast Neoplasms; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung | Details |
| Allitinib Tosylate | AST-6; ALS-1306; AST-1306 | Phase 2 Clinical | Shanghai Allist Pharmaceutical Technology Co Ltd | Breast Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Demupitamab | SCT-200 | Phase 2 Clinical | SinoCelltech Ltd | Ovarian Neoplasms; Biliary Tract Neoplasms; Solid tumours; Head and Neck Neoplasms; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Carcinoma; Carcinoma, Renal Cell; Triple Negative Breast Neoplasms; Neoplasms; Pancreatic Neoplasms; Colorectal Neoplasms; Gallbladder Neoplasms; Esophageal Squamous Cell Carcinoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| Cetuximab-IRDye800CW (University Medical Center Groningen) | Phase 2 Clinical | Umcg The University Medical Center Groningen | Rectal Neoplasms; Small Cell Lung Carcinoma; Penile Neoplasms; Lung Neoplasms | Details | |
| Selatinib Ditosilate | QLNC-120 | Phase 2 Clinical | Qilu Antibiotics (Linyi) Pharmaceutical Co Ltd, Qilu Pharmaceutical Co Ltd | Breast Neoplasms | Details |
| Pimurutamab | HLX-07 | Phase 2 Clinical | Shanghai Henlius Biotech Inc | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Stomach Neoplasms; Esophageal Neoplasms; Small Cell Lung Carcinoma; Carcinoma, Adenosquamous; Carcinoma, Basosquamous; Nasopharyngeal Carcinoma; Colorectal Neoplasms; Lung Neoplasms; Esophageal Squamous Cell Carcinoma; Carcinoma, Squamous Cell; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| NRC-2694 | NRC-2694; NRC-2694A; NRC-2694-A | Phase 2 Clinical | Natco Pharma | Carcinoma; Squamous Cell Carcinoma of Head and Neck | Details |
| Bafisontamab | FIT-013a; EMB-01 | Phase 2 Clinical | Shanghai Epimab Biotherapeutics, Inc | Biliary Tract Neoplasms; Liver Neoplasms; Carcinoid Tumor; Stomach Neoplasms; Neoplasms; Digestive System Neoplasms; Colorectal Neoplasms; Lung Neoplasms; Neoplasm Metastasis; Gastrointestinal Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| Recombinant anti-EGFR chimeric monoclonal antibody (Shanghai CP Guojian) | CPGJ-602; 602; CPGJ602; CPGJ 602 | Phase 2 Clinical | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | Colorectal Neoplasms | Details |
| GB-263 | GB-263; GB-263T | Phase 2 Clinical | Genor Biopharma Co Ltd | Solid tumours; Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| ORIC-114 | ORIC-114; VRN-07 | Phase 2 Clinical | Voronoi Inc | Solid tumours; Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| WJ-13404 | WJ-13404; JS-113; WJ-004 | Phase 2 Clinical | Wigen Biomedicine technology (Shanghai) Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| BDTX-1535 | BDTX-1535 | Phase 2 Clinical | Black Diamond Therapeutics Inc | Glioblastoma; Glioma; Carcinoma, Non-Small-Cell Lung | Details |
| Neratinib (Fukang (Shanghai) Health Technology) | CVL009; CVL-009 | Phase 2 Clinical | Solid tumours; Uterine Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| IBI-3001 | IBI-3001; IBI3001 | Phase 2 Clinical | Innovent Biologics(Suzhou) Co Ltd | Solid tumours | Details |
| TRX-221 | TRX-221 | Phase 2 Clinical | Therapex Corp | Carcinoma, Non-Small-Cell Lung | Details |
| E-EDV-D682 | PNU-15982; E-EDV-D682; EGFR-EDV-PNU-15982 | Phase 2 Clinical | Engeneic Ltd | Pancreatic Neoplasms | Details |
| IBI334 | IBI334; IBI-334 | Phase 2 Clinical | Innovent Biologics(Suzhou) Co Ltd | Solid tumours; Neoplasms | Details |
| DWP-708 | DWP-708 | Phase 2 Clinical | Daewoong Pharmaceutical Co Ltd | Acneiform Eruptions | Details |
| BBT-207 | BBT-207 | Phase 2 Clinical | Bridge Biotherapeutics Inc | Carcinoma, Non-Small-Cell Lung | Details |
| H-002 | H-002 | Phase 2 Clinical | Nanjing Hongyun Biological Technology Co Ltd | Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| DAJH-1050766 | DAJH1050766; DAJH-1050766 | Phase 2 Clinical | Carcinoma, Non-Small-Cell Lung | Details | |
| Anti-CTLA-4/PD-1 expressing EGFR-CAR-T | Phase 2 Clinical | Shanghai Cell Therapy Research Institute | Solid tumours | Details | |
| IAE-0972 | IAE-0972 | Phase 2 Clinical | SunHo (China) BioPharmaceutical Co Ltd | Solid tumours; Carcinoma, Hepatocellular | Details |
| Anti-EGFR CAR-T cell therapy (Beijing Pregene) | Phase 2 Clinical | Shenzhen Prekin Biopharmaceutical Co Ltd | Colorectal Neoplasms | Details | |
| BAY-2927088 | BAY-2927088; BAY2927088 | Phase 2 Clinical | Bayer AG, Broad Institute, Harvard University | Carcinoma, Non-Small-Cell Lung | Details |
| HS-10375 | HS-10375 | Phase 2 Clinical | Jiangsu Hansoh Pharmaceutical Group Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| SMET-12 | SMET-12 | Phase 2 Clinical | Zhejiang Shimai Pharmaceutical Co Ltd | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details |
| EO-1001 | NT-113; APL-122 | Phase 2 Clinical | Edison Pharmaceuticals Inc | Neoplasms | Details |
| TAK-186 | EGFR x CD3 COBRA; MVC-101; TAK-186 | Phase 2 Clinical | Takeda Pharmaceutical Co Ltd | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| AP-L1898 | JS111; WJ-002; AP-L1898 | Phase 2 Clinical | Wigen Biomedicine technology (Shanghai) Co Ltd | Small Cell Lung Carcinoma; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| BPI-361175 | BPI-361175 | Phase 2 Clinical | Betta Pharmaceuticals Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| CKD-702 | Phase 2 Clinical | Chong Kun Dang Pharmaceutical Corp | Stomach Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| REGN-7075 | REGN-7075 | Phase 2 Clinical | Solid tumours | Details | |
| JRF-103 | JRF103; JRF-103 | Phase 2 Clinical | Shenzhen Jinrui Foundation Biotechnology Co Ltd | Solid tumours | Details |
| Anti-EGFR-IL-dox (Swiss Group for Clinical Cancer Research) | Phase 2 Clinical | Schweizerische Arbeitsgemeinschaft Für Klinische Krebsforschung | Breast Neoplasms | Details | |
| FCN-411 | FCN-411 | Phase 2 Clinical | Shanghai Fosun Pharmaceutical (Group) Co Ltd | Small Cell Lung Carcinoma; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Epertinib | S-222611 | Phase 2 Clinical | Shionogi & Co Ltd | Neoplasms | Details |
| Pirotinib Hydrochloride | KBP-5209 | Phase 2 Clinical | Shandong Xuanzhu Pharmaceutical Technology Co Ltd | Solid tumours; Breast Neoplasms; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Humanized anti-EGFR monoclonal antibody (Hualan Biological Engineering/Henan shengming biotechnology) | Phase 2 Clinical | Henan Shengming Biotechnology Research Institute Co Ltd, Hualan Genetic Engineering Co Ltd | Colorectal Neoplasms | Details | |
| Anti-CD3/anti-EGFR -activated T cells (Barbara Ann Karmanos Cancer Institute) | Phase 2 Clinical | Barbara Ann Karmanos Cancer Institute, University Of Virginia | Glioblastoma; Pancreatic Neoplasms | Details | |
| Cetuximab biosimilar (Guilin Sanjin) | CDP-1; BC-001 | Phase 1 Clinical | Dragonboat Biopharmaceutical, Guilin Sanjin Pharmaceutical Co Ltd | Solid tumours; Neoplasms; Colorectal Neoplasms | Details |
| TQB3804 | TQB-3804 | Phase 1 Clinical | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| D2C7-based immunotoxins (Duke University) | D2C7-IT; scds-D2C7 -PE38KDEL; D2C7-(scdsFv)-PE38 KDEL | Phase 1 Clinical | Istari Oncology Inc, Duke University | Glioma | Details |
| Anti-EGFR CAR T-cell therapy (Seattle Children's Hospital) | EGFR-806 | Phase 1 Clinical | Seattle Children'S Hospital | Sarcoma, Synovial; Neoplasms, Germ Cell and Embryonal; Diffuse Intrinsic Pontine Glioma; Retinoblastoma; Neuroblastoma; Sarcoma; Neurofibrosarcoma; Sarcoma, Ewing; Osteosarcoma; Sarcoma, Clear Cell; Rhabdomyosarcoma; Central Nervous System Neoplasms; Wilms Tumor; Carcinoma; Rhabdoid Tumor; Hepatoblastoma; Desmoplastic Small Round Cell Tumor; Diffuse midline glioma; Solid tumours | Details |
| BPI-15086 | BPI-15086; BPI-15000 | Phase 1 Clinical | Betta Pharmaceuticals Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| Recombinant anti-human EGFR antibody/IL-10 bispecific Fc fusion protein(Dingfu Biotarget) | CmAb-(IL10)2; DF203; DF-203 | Phase 1 Clinical | Dingfu Biotarget Co Ltd | Solid tumours | Details |
| MP-0274 | DARPin-41; SPA-28; CME-114; CME-115; CME-118; CME-119; MP-0274 | Phase 1 Clinical | Molecular Partners Ag | Neoplasms | Details |
| SYN-004 (Synermore Biologics) | LR-004; SYN-004 | Phase 1 Clinical | Lonn Ryonn Pharma Ltd, Synermore Biologics (Suzhou) Co Ltd | Solid tumours; Carcinoma; Lymphoma, Large B-Cell, Diffuse; Colorectal Neoplasms | Details |
| KY-1701 | KY-1701 | Phase 1 Clinical | Jiangsu Kanion Pharmaceutical Co Ltd | Breast Neoplasms | Details |
| Simotinib Hydrochloride | AL-6802; SIM-6802 | Phase 1 Clinical | Jiangsu Simcere Pharmaceutical Co Ltd, Advenchen Laboratories Llc | Carcinoma, Non-Small-Cell Lung | Details |
| Double-deleted Vaccinia Virus Plus CD/ SMR | JX-929; vvDD-CDSR | Phase 1 Clinical | Sillajen Inc | Liver Neoplasms; Squamous Cell Carcinoma of Head and Neck; Pancreatic Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Melanoma | Details |
| WSD-0922 | WSD-0922; WSD0922 | Phase 1 Clinical | Wayshine Biopharm International Ltd | Glioblastoma; Neoplasms; Astrocytoma; Carcinoma, Non-Small-Cell Lung | Details |
| TQ-B3395 | TQ-B3395; CT-1495; TQ-B-3395 | Phase 1 Clinical | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd, Beijing Centaurus Biopharma Co Ltd | Neoplasms; Breast Neoplasms | Details |
| Antibody-drug nanocell conjugates (EnGeneIC) | EGFR-EDV-RRM1; EGFR-EDV-PLK; EGFR-EDV-Dox | Phase 1 Clinical | Engeneic Ltd | Glioblastoma | Details |
| Cetuximab biosimilar (Shanghai Henlius Biotech) | HLX-05; JZB-29; JZB-28 | Phase 1 Clinical | Shanghai Henlius Biotech Inc | Squamous Cell Carcinoma of Head and Neck; Colorectal Neoplasms | Details |
| Recombinant chimeric anti-EGFR monoclonal antibody (Zhejiang Xinwei Shengke Biotechnology) | Phase 1 Clinical | Zhejiang Xinweishengke Biotechnology Co Ltd | Esophageal Neoplasms | Details | |
| Anti-EGFR-bispecific antibody armed activated T-cell therapy (Memorial Sloan Kettering Cancer Center) | Phase 1 Clinical | Memorial Sloan Kettering Cancer Center | Pancreatic Neoplasms | Details | |
| [89Zr]Panitumumab | Phase 1 Clinical | Vanderbilt-Ingram Cancer Center, University Of Alabama At Birmingham, Stanford University | Squamous Cell Carcinoma of Head and Neck; Colonic Neoplasms; Pancreatic Neoplasms | Details | |
| Recombinant chimeric anti-EGFR monoclonal antibody (Qilu Pharmaceutical) | QL-1105 | Phase 1 Clinical | Qilu Pharmaceutical Co Ltd | Solid tumours | Details |
| EGFRvIII-CAR | Phase 1 Clinical | Duke University Medical Center | Glioblastoma | Details | |
| 14C-labeled poziotinib | Phase 1 Clinical | Spectrum Pharmaceuticals | Solid tumours | Details | |
| BB-101 (Blue Blood Biotech/National Cheng Kung University) | BB-101 | Phase 1 Clinical | National Cheng Kung University, Blue Blood Biotech Corp | Diabetic Foot | Details |
| Puvitinib | Phase 1 Clinical | Suzhou Teligene Ltd | Solid tumours | Details | |
| Betatinib | TL-512 | Phase 1 Clinical | Aspedia Llc, Suzhou Teligene Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| KSP-QRH-E3-IRDye800 | Phase 1 Clinical | University Of Michigan | Cholangiocarcinoma | Details | |
| Hemay-020 | Hemay-020 | Phase 1 Clinical | Tianjin Hemay Pharmaceutical Co Ltd, Hainan General Sanyang Pharmaceutical Co Ltd | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details |
| SGT-210 | SGT-210 | Phase 1 Clinical | Sol Gel Technologies Pte Ltd | Keratosis | Details |
| Epitinib Succinate | HMPL-813 | Phase 1 Clinical | Hutchison Medipharma Ltd | Solid tumours; Neoplasms; Glioblastoma; Carcinoma, Non-Small-Cell Lung | Details |
| ZN-e4 | ZN-e4; KP-673 | Phase 1 Clinical | Zentalis Pharmaceuticals LLC, Zeno Pharma | Carcinoma, Non-Small-Cell Lung | Details |
| LY-01010 | LY-01010 | Phase 1 Clinical | Luye Pharma Group Ltd | Breast Neoplasms | Details |
| PB-357 | PB-357 | Phase 1 Clinical | Pfizer Inc | Neoplasms | Details |
| ZSP-0391 | ZSP-0391 | Phase 1 Clinical | Wuxi Apptec Co Ltd, Guangdong Zhongsheng Pharmaceutical Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| Neptinib Di-P-methylbenzenesulfonate | Phase 1 Clinical | Shenzhen Neptunus Pharmaceutical Research Institute Co Ltd | Stomach Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| QRH-882260 | QRH-882260 | Phase 1 Clinical | University Of Michigan | Cholangiocarcinoma | Details |
| Serclutamab talirine | ABBV-321 | Phase 1 Clinical | Abbvie Inc | Neoplasms | Details |
| Recombinant anti-EGFR chimeric antibody (Harbin Pharmaceutical) | Phase 1 Clinical | Harbin Pharmaceutical Group Holding Co Ltd | Colorectal Neoplasms | Details | |
| Diakine-DK2 10 (EGFR) | DK210; DK-2-10 | Phase 1 Clinical | Deka Biosciences Inc | Kidney Neoplasms; Solid tumours; Head and Neck Neoplasms; Carcinoma, Renal Cell; Neoplasms; Pancreatic Neoplasms; Skin Neoplasms; Colorectal Neoplasms; Genital Neoplasms, Female; Carcinoma, Non-Small-Cell Lung | Details |
| HLX-42 | HLX-42; HLX42 | Phase 1 Clinical | Hanlin Biotechnology Co Ltd | Solid tumours; Neoplasms | Details |
| CMAB-017 | CMAB-017 | Phase 1 Clinical | Taizhou Mabtech Pharmaceutical Co Ltd | Solid tumours | Details |
| JY-016 | JY-016 | Phase 1 Clinical | Beijing Jingyitaixiang Technology Development Co Ltd, Beijing Eastern Biotech Co Ltd | Solid tumours; Colorectal Neoplasms; Lung Neoplasms | Details |
| PF-08046052 | SGN-EGFRd2; LAVA-1223 | Phase 1 Clinical | Vu University Medical Center, Lava Therapeutics NV | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Colorectal Neoplasms; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung | Details |
| CM-93 | CM-93 | Phase 1 Clinical | Shanghai Crimson Pharmaceutical Co Ltd | Glioblastoma | Details |
| BLU-701 | BLU-701; BLU 701 | Phase 1 Clinical | Blueprint Medicines Corp | Carcinoma, Bronchogenic; Carcinoma; Thoracic Neoplasms; Bronchial Neoplasms; Neoplasms; Respiratory Tract Diseases; Lung Diseases; Respiratory Tract Neoplasms; Neoplasms, Nerve Tissue; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma | Details |
| CX-904 | CX-904 | Phase 1 Clinical | Amgen Inc, Cytomx Therapeutics Inc | Solid tumours; Neoplasms | Details |
| Cetuximab biosimilar (Humanwell Healthcare) | Phase 1 Clinical | Humanwell Healthcare (Group) Co Ltd | Colorectal Neoplasms | Details | |
| Sirotinib Maleate | XZP-5491 | Phase 1 Clinical | Shandong Xuanzhu Pharmaceutical Technology Co Ltd | Stomach Neoplasms; Carcinoma, Squamous Cell; Carcinoma, Non-Small-Cell Lung | Details |
| Tomuzotuximab | GT-MAB-5.2-GEX | Phase 1 Clinical | Glycotope Gmbh | Solid tumours; Squamous Cell Carcinoma of Head and Neck | Details |
| YL-221 | YL221; YL-221 | Phase 1 Clinical | MediLink Therapeutics Co Ltd | Solid tumours | Details |
| [14C]-PLB1004 | [14C]-PLB1004 | Phase 1 Clinical | Beijing Avistone Pharmaceuticals Biotechnology Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| FPI-2107 | [111In]-FPI-2107 | Phase 1 Clinical | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Colorectal Neoplasms; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung | Details | |
| FPI-2053 | FPI-2053 | Phase 1 Clinical | Astrazeneca Plc | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Colorectal Neoplasms; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung | Details |
| CPO-301 | CPO301; CPO-301 | Phase 1 Clinical | CSPC Pharmaceutical Group Ltd | Solid tumours; Neoplasms; Lung Neoplasms | Details |
| FPI-2068 | [225Ac]-FPI-2068; FPI-2068 | Phase 1 Clinical | Astrazeneca Plc | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Neoplasms; Colorectal Neoplasms; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung | Details |
| HS-20117 | HS-20117; PM1080; PM-1080 | Phase 1 Clinical | Biotheus Inc | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details |
| SYS-6010 | SYS6010; SYS-6010 | Phase 1 Clinical | Jushi Biopharmaceutical Co Ltd | Solid tumours | Details |
| SNC-109 | SNC109; SNC-109 | Phase 1 Clinical | Shanghai Simnova Biotechnology Co Ltd | Glioblastoma | Details |
| HSK-40118 | HSK40118; HSK-40118 | Phase 1 Clinical | Haisco Pharmaceutical Group Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| AZD9592 | AZD-9592; AZD9592 | Phase 1 Clinical | Astrazeneca Plc | Solid tumours; Head and Neck Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| JANX-008 | EGFR-TRACTr; JANX008; JANX-008 | Phase 1 Clinical | Janux Therapeutics Inc | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| TAVO-412 | TAVO-412 | Phase 1 Clinical | Tavotek Biotherapeutics (Hong Kong) Ltd, Tuochuang Biotechnology (Jiangsu) Co Ltd | Solid tumours; Neoplasms | Details |
| NX-019 | NX-019 | Phase 1 Clinical | Nalo Therapeutics Inc | Neoplasms | Details |
| QLH-11811 | QLH-11811; QLH11811 | Phase 1 Clinical | Qilu Pharmaceutical Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| Losatuxizumab vedotin | ABBV-221; ABBV221 | Phase 1 Clinical | Abbvie Inc | Neoplasms | Details |
| EGFR/B7H3 CAR-T | EGFR/B7H3 CAR-T | Phase 1 Clinical | Second Affiliated Hospital Of Guangzhou Medical University | Triple Negative Breast Neoplasms; Lung Neoplasms | Details |
| BB-1705 | BB-1705 | Phase 1 Clinical | Bliss Biopharmaceutical (Hangzhou) Co Ltd | Solid tumours; Neoplasms; Neoplasm Metastasis | Details |
| CART-EGFR-IL13Ra2 | CART-EGFR-IL13Ra2; CAR-T-EGFR-IL-13-Ra-2 | Phase 1 Clinical | University Of Pennsylvania | Glioblastoma | Details |
| EGFR IL12 CART | Phase 1 Clinical | Shenzhen Prekin Biopharmaceutical Co Ltd | Colorectal Neoplasms | Details | |
| ERAS-801 | ERAS-801 | Phase 1 Clinical | Regents Of The University Of California | Glioblastoma | Details |
| NIP-142 | NIP-142 | Phase 1 Clinical | National Institutes Of Pharmaceutical Research And Development Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| TAS-2940 | TAS-2940 | Phase 1 Clinical | Taiho Oncology Inc | Solid tumours; Glioblastoma; Breast Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| [111In] Panitumumab | Phase 1 Clinical | Stanford University | Head and Neck Neoplasms; Squamous Cell Carcinoma of Head and Neck | Details | |
| BC-3448 | BC3448; BC-3448 | Phase 1 Clinical | Solid tumours | Details | |
| ABBV-637 | ABBV-637 | Phase 1 Clinical | Abbvie Inc | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details |
| M-1231 | M-1231 | Phase 1 Clinical | Emd Serono Research & Development Institute Inc, Merck Serono | Solid tumours; Esophageal Neoplasms; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung | Details |
| HLX-35 | HLX-35 | Phase 1 Clinical | Shanghai Henlius Biologics Co Ltd | Solid tumours; Carcinoma, Squamous Cell; Carcinoma, Non-Small-Cell Lung | Details |
| WTS-004 | WTS-004 | Phase 1 Clinical | Hangzhou Wutong Tree Pharmaceutical Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| ES-072 | ES-072 | Phase 1 Clinical | Zhejiang Bosheng Pharmaceutical Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| ZZ-06 | ZZ-06 | Phase 1 Clinical | Changchun Intellicrown Pharmaceutical Co Ltd | Solid tumours | Details |
| ABY-029 | ABY-029 | Phase 1 Clinical | Dartmouth College, Affibody Ab, Li-Cor Bioscience | Head and Neck Neoplasms; Sarcoma; Glioma | Details |
| Lifirafenib | BeiGene-283; BGB-283 | Phase 1 Clinical | Beigene Ltd | Solid tumours | Details |
| Cetuximab biosimilar (Enzene Biosciences) | Clinical | Enzene Biosciences Ltd | Carcinoma, Squamous Cell | Details | |
| VRN-11 | VRN11 | Clinical | Voronoi Inc | Carcinoma, Non-Small-Cell Lung | Details |
This web search service is supported by Google Inc.







