
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions

This protein carries a polyhistidine tag at the C-terminus.
The protein has a calculated MW of 85.9 kDa.
PE
Excitation Wavelength: 488 nm / 561 nm
Emission Wavelength: 575 nm
Lyophilized from 0.22 μm filtered solution in PBS, 0.5% BSA, pH7.4. Normally trehalose is added as protectant before lyophilization.
Contact us for customized product form or formulation.
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please protect from light and avoid repeated freeze-thaw cycles.
This product is stable after storage at:
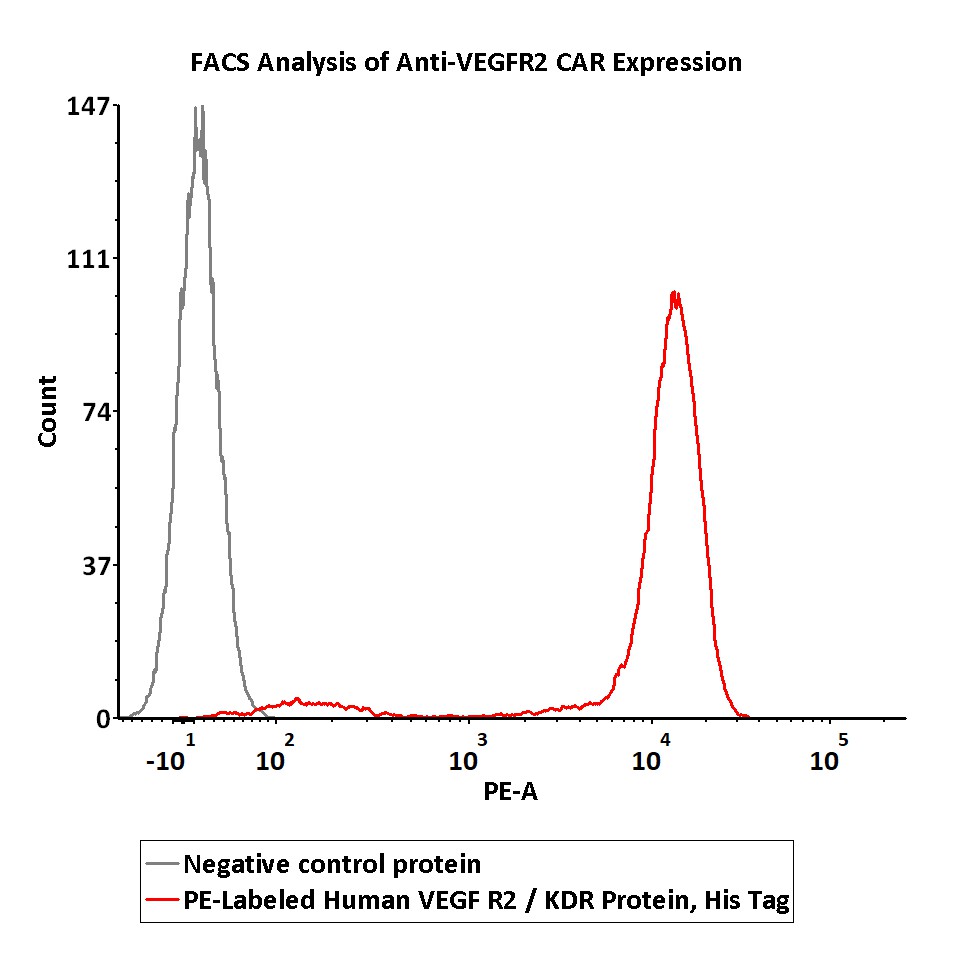
5e5 of anti-VEGFR2 CAR-293 cells were stained with 100 μL of 1:25 dilution (4 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human VEGF R2, His Tag (Cat. No. KDR-HP227) and negative control protein respectively. PE signal was used to evaluate the binding activity (QC tested).
Please contact us via TechSupport@acrobiosystems.com if you have any question on this product.

Explore our catalog of therapeutic antibody solutions to find the right products for you! We are dedicated to delivering solutions designed to help you drive innovation and push the boundaries of what therapeutic antibodies can be.

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

Organoid Toolbox is a collection of organoid solutions including ready-to-use organoids, organoid differentiation kits, and a variety of services to accelerate the progress of your drug development project.

ACROBiosystems developed a series of GMP grade cytokines under the GMP grade quality management system. Those products are all suitable for T/NK cell generation, activation, and proliferation in cell therapy research.

50+ targets designed for CAR detection, including PE/FITC/biotin labeled proteins. The key reagents for CD19 and BCMA were FDA DMF filed which can support your IND, NDA and BLA process.

Full length multi-pass TPs with stabilized structure and high bioactivity for immunization, antibody screening, cell based assay and CAR detection, including hot CD20, Claudin 18.2, CD133, GPRC5D,CCR8, CCR5, etc.

GMP grade cytokines, reagents for cell activation, gene edition, DNA/RNA removal, etc. Particularly focus on product design, quality control and solution-based support to link each phase of your cell and gene therapy journey.

A series of immune checkpoints including classic co-inhibitory and co-stimulatory receptors. The comprehensive catalog contains 100+ targets with various species and tags, and the high-quality proteins are in good batch-to-batch consistency.

To meet the needs of ADCs development, ACROBiosystems can provide: A variety of high-quality target proteins; MMPs/Cathepsin/uPA for cleavable linker; Anti-payload antibodies & anti-idiotypic antibodies for immunogenicity and PK analysis; SPR/BLI analytical and ADA development service.

Comprehensive collection of Fc receptor proteins, including their common variants, which can help expedite your antibody development.

Comprehensive cytokine targets including interleukins, growth factors, chemokines, TNFs, etc. are expressed by HEK293 to ensure their natural structure. Their high purity is verified by SDS-PAGE/HPLC/SEC-MALS and high bioactivity is verified by ELISA/SPR/BLI.

Aneuro provides innovative solutions for neuroscience research. Recombinant proteins, neural factors, pre-formed fibrils, electrophysiological electrodes, as well as Organoid Toolbox all in Aneuro aiming to advance neuroscience research, develop therapeutic interventions, and improve diagnostic methods for neurological diseases.
| English Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Anlotinib Dihydrochloride | AL-3818 | Approved | Advenchen Laboratories Llc, Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 福可维 | Mainland China | Carcinoma, Non-Small-Cell Lung | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 2018-05-08 | Sarcoma, Alveolar Soft Part; Bile Duct Diseases; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Peritoneal Neoplasms; Hepatic Insufficiency; Bone Neoplasms; Urologic Neoplasms; Fallopian Tube Neoplasms; Thyroid Neoplasms; Endometrial Neoplasms; Medullary thyroid cancer (MTC); Gallbladder Neoplasms; Glioma; Lung Neoplasms; Esophageal Squamous Cell Carcinoma; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Osteoma; Melanoma; Carcinoma, Hepatocellular; Carcinoma, Ovarian Epithelial; Leiomyosarcoma; Solid tumours; Drug-Related Side Effects and Adverse Reactions; Biliary Tract Neoplasms; Head and Neck Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Thoracic Neoplasms; Carcinoma, Renal Cell; Ovarian Neoplasms; Small Cell Lung Carcinoma; Neoplasms; Lymphoma, Large B-Cell, Diffuse; Sarcoma, Synovial; Neuroendocrine Tumors; Lung Diseases, Interstitial; Liver Diseases; Sarcoma; Nasopharyngeal Carcinoma | Details |
| Lenvatinib Mesylate | MK-7902; ER-203492-00; E-7080 | Approved | Eisai Co Ltd | Kisplyx, Lenvima, Lenvima/Kisplyx, 乐卫玛 | United States | Thyroid Neoplasms | Eisai Inc | 2015-02-13 | Carcinoma, Adenoid Cystic; Paraganglioma; Melanoma; Thyroid Cancer, Papillary; Carcinoma, Hepatocellular; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Glioma; Lymphoma; Endometrial Neoplasms; Thyroid Neoplasms; Hepatic Insufficiency; Breast Neoplasms; Cholangiocarcinoma; Osteosarcoma; Solid tumours; Neuroendocrine Tumors; Adenocarcinoma, Follicular; Liver Diseases; Thyroid Carcinoma, Anaplastic; Adenocarcinoma of Lung; Kidney Diseases; Neoplasms; Pheochromocytoma; Esophageal Neoplasms; Renal Insufficiency; Carcinoma, Renal Cell; Liver Neoplasms; Ovarian Neoplasms; Biliary Tract Neoplasms | Details |
| Axitinib | PF-01367866; AG-13736; AG-013736 | Approved | Pfizer Inc | 英立达, Inlyta | United States | Carcinoma, Renal Cell | Pf Prism Cv | 2012-01-27 | Lung Neoplasms; Carcinoma, Adenoid Cystic; Prostatic Neoplasms, Castration-Resistant; Breast Neoplasms; Sarcoma; Prostatic Neoplasms; Cholangiocarcinoma; Hepatic Insufficiency; Colorectal Neoplasms; Thyroid Neoplasms; Neuroendocrine Tumors; Leukemia, Myeloid, Acute; Carcinoma, Pancreatic Ductal; Lymphoma; Carcinoma, Hepatocellular; Adenocarcinoma; Carcinoma, Non-Small-Cell Lung; Melanoma; Paraganglioma; Myelodysplastic Syndromes; Ovarian Neoplasms; Solid tumours; Carcinoma, Renal Cell; Carcinoma; Pheochromocytoma; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Carcinoid Tumor; Kidney Neoplasms; Neoplasms; Nasopharyngeal Neoplasms; Adrenal Cortex Neoplasms; Skin Neoplasms; Pancreatic Neoplasms; Glioblastoma; Mesothelioma; Carcinoma, Ductal | Details |
| Regorafenib | DAST; BAY-73-4506 | Approved | Bayer AG | Stivarga, Resihance | United States | Colorectal Neoplasms | Bayer Healthcare Pharmaceuticals Inc | 2012-09-27 | Fallopian Tube Neoplasms; Osteosarcoma; Sarcoma, Ewing; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Peritoneal Neoplasms; Bone Neoplasms; Bile Duct Neoplasms; Thymoma; Thyroid Neoplasms; Leukemia, Myeloid, Acute; Gastrinoma; Lung Neoplasms; Esophageal adenocarcinoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Melanoma; Gastrointestinal Neoplasms; Somatostatinoma; Adenocarcinoma; Neoplasm Metastasis; Meningioma; Neoplasms; Solid tumours; Ovarian Neoplasms; Rectal Neoplasms; Carcinoma, Renal Cell; Hemangiosarcoma; Carcinoid Tumor; Insulinoma; Carcinoma, Islet Cell; Stomach Neoplasms; Esophageal Neoplasms; Liver Neoplasms; Carcinoma, Transitional Cell; Colonic Neoplasms; Pancreatic Neoplasms; Glioblastoma; Carcinoma, Ovarian Epithelial; Adenoma; Glucagonoma; Carcinoma, Adenoid Cystic; Sarcoma | Details |
| Nintedanib Esylate | BIBF-1120 | Approved | C.H. Boehringer Sohn Ag & Co. Kg | Ofev, Vargatef | United States | Idiopathic Pulmonary Fibrosis | Boehringer Ingelheim Gmbh | 2014-10-15 | Lung Neoplasms; Breast Neoplasms; Prostatic Neoplasms; Adenocarcinoma, Clear Cell; systemic sclerosis-associated interstitial lung disease; Colorectal Neoplasms; Peritoneal Neoplasms; Gliosarcoma; Hepatic Insufficiency; Astrocytoma; Genital Neoplasms, Female; Silicosis; Sarcoma; Carcinoma, Squamous Cell; Fallopian Tube Neoplasms; Appendiceal Neoplasms; Leukemia, Myeloid, Acute; Uterine Neoplasms; Endometrial Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Adenocarcinoma; Carcinoma, Endometrioid; Scleroderma, Systemic; Telangiectasia, Hereditary Hemorrhagic; Solid tumours; Rejection of lung transplantation; Carcinoma, Renal Cell; Radiation Pneumonitis; Esophageal Neoplasms; Carcinoid Tumor; Endometrial Stromal Tumors; Idiopathic Pulmonary Fibrosis; Neoplasms; Ovarian Neoplasms; Glioblastoma; Colonic Neoplasms; Small Cell Lung Carcinoma; Pulmonary Fibrosis; Lung Diseases, Interstitial; Oligodendroglioma; Multiple Myeloma; Mesothelioma; Asbestosis; Neuroendocrine Tumors | Details |
| Vorolanib | X-82; EYP-1901; CM-082 | Approved | Tyrogenex Inc | 伏美纳 | Mainland China | Carcinoma, Renal Cell | Betta Pharmaceuticals Co Ltd | 2023-06-07 | Diabetic macular oedema; Carcinoma, Non-Small-Cell Lung; Melanoma; Macular Degeneration; Carcinoma, Hepatocellular; Diabetic Retinopathy; Choroidal Neovascularization; Adenocarcinoma; Retinal Degeneration; Leukemia, Myeloid, Acute; Eye Diseases; Solid tumours; Wet Macular Degeneration; Retinal Diseases; Small Cell Lung Carcinoma; Neoplasms; Pancreatic Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Carcinoma, Renal Cell; Kidney Neoplasms | Details |
| Tivozanib | Kil-8951; AV-951; KRN-951; ASP-4130; KHK-4951; KHK4951; UNII-172030934T | Approved | Kyowa Hakko Kirin Co Ltd | Fotivda, FOTIVDA | EU | Carcinoma, Renal Cell | Recordati Netherlands BV | 2017-08-24 | Colorectal Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Gastrointestinal Neoplasms; Macular Degeneration; Fallopian Tube Neoplasms; Metastatic breast cancer; Peritoneal Neoplasms; Hepatic Insufficiency; Bile Duct Neoplasms; Biliary Tract Neoplasms; Sarcoma; Breast Neoplasms; Cholangiocarcinoma; Carcinoma, Ovarian Epithelial; Triple Negative Breast Neoplasms; Carcinoma, Renal Cell; Ovarian Neoplasms; Liver Neoplasms; Solid tumours | Details |
| Apatinib Mesylate | YN-968D1 | Approved | Advenchen Laboratories Llc | 艾坦, Aitan | Mainland China | Stomach Neoplasms | Jiangsu shengdi Medicine Co Ltd | 2014-10-17 | Nasopharyngeal Neoplasms; Adenocarcinoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Lung Neoplasms; Fallopian Tube Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Nasopharyngeal Carcinoma; Breast Neoplasms; Carcinoma, Adenoid Cystic; Head and Neck Neoplasms; Triple Negative Breast Neoplasms; Carcinoma, Ovarian Epithelial; Neoplasms; Esthesioneuroblastoma, Olfactory; Esophageal Neoplasms; Stomach Neoplasms; Solid tumours; Ovarian Neoplasms; Liver Neoplasms; Biliary Tract Neoplasms | Details |
| Ripretinib | DCC-2618 | Approved | Deciphera | Qinlock, 擎乐 | United States | Gastrointestinal Stromal Tumors | Deciphera Pharmaceuticals Llc | 2020-05-15 | Neoplasms; Mastocytosis, Systemic; Gastrointestinal Stromal Tumors | Details |
| Ramucirumab | IMC-1121; IMC-1C11; LY-3009806; IMC-1121-B; BLA-125477 | Approved | Dyax Pharma | Cyramza | United States | Gastrointestinal Stromal Tumors | Eli Lilly And Company | 2014-04-21 | Breast Neoplasms; Melanoma; Carcinoma, Hepatocellular; Adenocarcinoma; Carcinoma, Non-Small-Cell Lung; Fallopian Tube Neoplasms; Ureteral Neoplasms; Urologic Neoplasms; Peritoneal Neoplasms; Gastrointestinal Stromal Tumors; Colorectal Neoplasms; Prostatic Neoplasms; Urethral Neoplasms; Liver Neoplasms; Pancreatic Neoplasms; Colonic Neoplasms; Carcinoma, Transitional Cell; Neoplasms; Carcinoma, Renal Cell; Rectal Neoplasms; Esophageal Neoplasms; Carcinoid Tumor; Stomach Neoplasms; Biliary Tract Neoplasms; Ovarian Neoplasms; Solid tumours | Details |
| Sunitinib Malate | PNU-290940AD; PHA-290940AD; SCAI-003; GB-102; PNU-290940; SU-011248-L-malate salt; PHA-290940; SU-010398; SU-11248 | Approved | Pfizer Inc | 索坦, Sutent | United States | Carcinoma, Renal Cell; Gastrointestinal Stromal Tumors | Cppi Cv | 2006-01-26 | Solid tumours; Fibromatosis, Aggressive; Ovarian Neoplasms; Kidney Neoplasms; Leukemia, Myelogenous, Chronic; Head and Neck Neoplasms; Leiomyosarcoma; Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; HIV Infections; Leukemia, Myeloid, Accelerated Phase; Leukemia; Fibrosarcoma; Teratoma; Liver Neoplasms; Ependymoma; Lymphoma, T-Cell, Peripheral; Intestinal Neoplasms; Histiocytoma, Malignant Fibrous; Carcinoma, Renal Cell; Hemangioblastoma; Carcinoma, Islet Cell; Carcinoma; Pheochromocytoma; Stomach Neoplasms; Pelvic Neoplasms; Esophageal Neoplasms; Leukemia, Hairy Cell; Polycythemia Vera; Abdominal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Thoracic Neoplasms; Pancreatic neuroendocrine tumors (pNET); Carcinoma, Ovarian Epithelial; Glioblastoma; Neurofibromatoses; Small Cell Lung Carcinoma; Adenoma, Islet Cell; Carcinoma, Transitional Cell; Pancreatic Neoplasms; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Myelodysplastic Syndromes; Hodgkin Disease; Leukemia, Myelomonocytic, Chronic; Carcinoma, Papil | Details |
| Pazopanib Hydrochloride | GSK-786034; GW-786034B; SB-786034; GW-786034 | Approved | Glaxosmithkline Plc, Novartis Pharma Ag | 维全特, Armala, Votrient, Patorma | United States | Carcinoma, Renal Cell; Sarcoma | Novartis Pharma Ag | 2009-10-19 | Uterine Cervical Diseases; Fallopian Tube Neoplasms; Lung Neoplasms; Uterine Neoplasms; Choriocarcinoma; Lymphoma; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Carcinoma, Mucoepidermoid; Gliosarcoma; Genital Neoplasms, Female; Leukemia, Myeloid, Acute; Peritoneal Neoplasms; Brain Neoplasms; Urethral Neoplasms; Medullary thyroid cancer (MTC); Chondrosarcoma, Extraskeletal Myxoid; Prostatic Neoplasms; Osteosarcoma; Neuroblastoma; Sarcoma; Breast Neoplasms; Carcinoma, Hepatocellular; Uterine Cervical Neoplasms; Breast Neoplasms, Male; Neoplasm Metastasis; von Hippel-Lindau Disease; Thyroid Cancer, Papillary; Gastrointestinal Neoplasms; Paraganglioma; Endodermal Sinus Tumor; Neoplasms, Germ Cell and Embryonal; Melanoma; Gastrinoma; Macular Degeneration; Carcinoma, Non-Small-Cell Lung; Thyroid Neoplasms; Carcinoma, Squamous Cell; Carcinoma, Small Cell; Carcinoma, Embryonal; Germinoma; Glioma; Carcinoma, Neuroendocrine; Epistaxis; Carcinoma, Ovarian Epithelial; Neoplasms; Squamous Cell Carcinoma of Head a | Details |
| Cabozantinib S-malate | XL-184; BMS-907351 | Approved | Exelixis Inc | Cometriq, Cabometyx | United States | Carcinoma, Neuroendocrine; Thyroid Neoplasms | Exelixis Inc | 2012-11-29 | Urethral Neoplasms; Leukemia, Myeloid, Acute; Thyroid Neoplasms; Gliosarcoma; Colorectal Neoplasms; Astrocytoma; Hepatic Insufficiency; Peritoneal Neoplasms; Bile Duct Neoplasms; Sarcoma, Clear Cell; Uterine Neoplasms; Adenocarcinoma, Clear Cell; Sarcoma, Ewing; Carcinoma, Adenosquamous; Breast Neoplasms; Neurofibroma, Plexiform; Osteosarcoma; Prostatic Neoplasms; Sarcoma; Medullary thyroid cancer (MTC); Carcinoma, Hepatocellular; Melanoma; Paraganglioma; Meningioma; Neoplasms, Germ Cell and Embryonal; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Carcinoma, Endometrioid; Thyroid Cancer, Papillary; Brain Neoplasms; Carcinoma, Neuroendocrine; Sarcoma, Alveolar Soft Part; Lymphoma; Fallopian Tube Neoplasms; Brain metastases; Glioma; Endometrial Neoplasms; Carcinoma, Squamous Cell; Carcinoid Tumor; Glioblastoma; Skin Neoplasms; Neoplasms; Carcinoma, Papillary; Hepatoblastoma; Pheochromocytoma; Pain; Rejection of liver transplantation; Carcinoma, Renal Cell; Pancreatic neuroendocrine tumors | Details |
| Fruquintinib | HMPL-013; TAK-113 | Approved | Hutchison Medipharma Ltd | Elunate, FRUZAQLA, Aiyoute, 爱优特 | Mainland China | Colorectal Neoplasms | Hutchison Medipharma Ltd | 2018-09-04 | Small Cell Lung Carcinoma; Carcinoma, Non-Small-Cell Lung; Endometrial Neoplasms; Lung Neoplasms; Hepatic Insufficiency; Colorectal Neoplasms; Breast Neoplasms; Sarcoma; Kidney Diseases; Neoplasms; Solid tumours; Triple Negative Breast Neoplasms; Pancreatic Neoplasms; Colonic Neoplasms; Rectal Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Renal Cell; Biliary Tract Neoplasms | Details |
| Sorafenib Tosylate | NSC-724772; BAY-43-0006; BAY-43-9006; BAY-54-9085 | Approved | Onyx Pharmaceuticals Inc | Nexavar, 多吉美 | United States | Carcinoma, Renal Cell | Bayer Healthcare Pharmaceuticals Inc | 2005-12-01 | Liver Neoplasms; Kidney Neoplasms; Recurrence; Ovarian Neoplasms; Leukemia, Myelogenous, Chronic; Lymphoma, T-Cell, Peripheral; Leukemia, Erythroblastic, Acute; Rhabdomyosarcoma; Lymphoma, B-Cell, Marginal Zone; Fibromatosis, Aggressive; Head and Neck Neoplasms; Solid tumours; Leiomyosarcoma; Leukemia, Myeloid; Carcinoma, Renal Cell; Carcinoma; Vipoma; Esophageal Neoplasms; Hemangiosarcoma; Squamous Cell Carcinoma of Head and Neck; Rectal Neoplasms; Carcinoid Tumor; Histiocytoma, Malignant Fibrous; Carcinoma, Islet Cell; Insulinoma; Stomach Neoplasms; Neoplasms; Kidney Diseases; Small Cell Lung Carcinoma; Carcinoma, Transitional Cell; Carcinoma, Verrucous; Thyroid Carcinoma, Anaplastic; Myelodysplastic Syndromes; Glioblastoma; Pancreatic Neoplasms; Leukemia, Myelomonocytic, Chronic; Carcinoma, Ovarian Epithelial; Leukemia, Myelomonocytic, Acute; Wilms Tumor; Lymphomatoid Granulomatosis; Colonic Neoplasms; Hypertension, Portal; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Large-Cell, Immunoblastic; Multiple Endo | Details |
| Vandetanib | AZD-6474; CH-331; ZD-6474 | Approved | Astrazeneca Plc | Zactima, Caprelsa | United States | Carcinoma, Neuroendocrine; Thyroid Neoplasms | Genzyme Corp | 2011-04-06 | Lung Neoplasms; Neuroblastoma; Medullary thyroid cancer (MTC); Prostatic Neoplasms; Urethral Neoplasms; Peritoneal Neoplasms; Gliosarcoma; Colorectal Neoplasms; Ureteral Neoplasms; Gastrointestinal Stromal Tumors; Diffuse Intrinsic Pontine Glioma; Lymphoproliferative Disorders; Astrocytoma; Thyroid Neoplasms; Pleural Effusion; Carcinoma, Squamous Cell; Lymphoma; Fallopian Tube Neoplasms; Multiple Endocrine Neoplasia Type 2b; Glioma; Carcinoma, Neuroendocrine; Carcinoma, Non-Small-Cell Lung; Paraganglioma; Precancerous Conditions; Neoplasm Metastasis; Adenocarcinoma; von Hippel-Lindau Disease; Carcinoma, Hepatocellular; Anus Neoplasms; Biliary Tract Neoplasms; Solid tumours; Kidney Neoplasms; Intestinal Neoplasms; Ovarian Neoplasms; Pheochromocytoma; Stomach Neoplasms; Esophageal Neoplasms; Carcinoma, Renal Cell; Carcinoma; Squamous Cell Carcinoma of Head and Neck; Rectal Neoplasms; Abdominal Neoplasms; Head and Neck Neoplasms; Small Cell Lung Carcinoma; Carcinoma, Transitional Cell; Pancreatic Neoplasms; Neop | Details |
| Midostaurin | PKC-412; PKC-412A; CGP-41231; CGP-41251 | Approved | Novartis Pharma Ag | Rydapt | United States | Mastocytosis, Systemic; Leukemia, Mast-Cell; Hematologic Neoplasms; Leukemia, Myeloid, Acute | Novartis Pharmaceuticals Corp | 2017-04-28 | Leukemia; Hematologic Neoplasms; Mastocytosis, Systemic; Myelodysplastic Syndromes; Leukemia, Mast-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Hepatic Insufficiency; Leukemia, Myeloid, Acute; Mastocytosis | Details |
| Anlotinib Dihydrochloride | AL-3818 | Approved | Advenchen Laboratories Llc, Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 福可维 | Mainland China | Carcinoma, Non-Small-Cell Lung | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 2018-05-08 | Sarcoma, Alveolar Soft Part; Bile Duct Diseases; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Peritoneal Neoplasms; Hepatic Insufficiency; Bone Neoplasms; Urologic Neoplasms; Fallopian Tube Neoplasms; Thyroid Neoplasms; Endometrial Neoplasms; Medullary thyroid cancer (MTC); Gallbladder Neoplasms; Glioma; Lung Neoplasms; Esophageal Squamous Cell Carcinoma; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Osteoma; Melanoma; Carcinoma, Hepatocellular; Carcinoma, Ovarian Epithelial; Leiomyosarcoma; Solid tumours; Drug-Related Side Effects and Adverse Reactions; Biliary Tract Neoplasms; Head and Neck Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Thoracic Neoplasms; Carcinoma, Renal Cell; Ovarian Neoplasms; Small Cell Lung Carcinoma; Neoplasms; Lymphoma, Large B-Cell, Diffuse; Sarcoma, Synovial; Neuroendocrine Tumors; Lung Diseases, Interstitial; Liver Diseases; Sarcoma; Nasopharyngeal Carcinoma | Details |
| Lenvatinib Mesylate | MK-7902; ER-203492-00; E-7080 | Approved | Eisai Co Ltd | Kisplyx, Lenvima, Lenvima/Kisplyx, 乐卫玛 | United States | Thyroid Neoplasms | Eisai Inc | 2015-02-13 | Carcinoma, Adenoid Cystic; Paraganglioma; Melanoma; Thyroid Cancer, Papillary; Carcinoma, Hepatocellular; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Glioma; Lymphoma; Endometrial Neoplasms; Thyroid Neoplasms; Hepatic Insufficiency; Breast Neoplasms; Cholangiocarcinoma; Osteosarcoma; Solid tumours; Neuroendocrine Tumors; Adenocarcinoma, Follicular; Liver Diseases; Thyroid Carcinoma, Anaplastic; Adenocarcinoma of Lung; Kidney Diseases; Neoplasms; Pheochromocytoma; Esophageal Neoplasms; Renal Insufficiency; Carcinoma, Renal Cell; Liver Neoplasms; Ovarian Neoplasms; Biliary Tract Neoplasms | Details |
| Axitinib | PF-01367866; AG-13736; AG-013736 | Approved | Pfizer Inc | 英立达, Inlyta | United States | Carcinoma, Renal Cell | Pf Prism Cv | 2012-01-27 | Lung Neoplasms; Carcinoma, Adenoid Cystic; Prostatic Neoplasms, Castration-Resistant; Breast Neoplasms; Sarcoma; Prostatic Neoplasms; Cholangiocarcinoma; Hepatic Insufficiency; Colorectal Neoplasms; Thyroid Neoplasms; Neuroendocrine Tumors; Leukemia, Myeloid, Acute; Carcinoma, Pancreatic Ductal; Lymphoma; Carcinoma, Hepatocellular; Adenocarcinoma; Carcinoma, Non-Small-Cell Lung; Melanoma; Paraganglioma; Myelodysplastic Syndromes; Ovarian Neoplasms; Solid tumours; Carcinoma, Renal Cell; Carcinoma; Pheochromocytoma; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Carcinoid Tumor; Kidney Neoplasms; Neoplasms; Nasopharyngeal Neoplasms; Adrenal Cortex Neoplasms; Skin Neoplasms; Pancreatic Neoplasms; Glioblastoma; Mesothelioma; Carcinoma, Ductal | Details |
| Regorafenib | DAST; BAY-73-4506 | Approved | Bayer AG | Stivarga, Resihance | United States | Colorectal Neoplasms | Bayer Healthcare Pharmaceuticals Inc | 2012-09-27 | Fallopian Tube Neoplasms; Osteosarcoma; Sarcoma, Ewing; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Peritoneal Neoplasms; Bone Neoplasms; Bile Duct Neoplasms; Thymoma; Thyroid Neoplasms; Leukemia, Myeloid, Acute; Gastrinoma; Lung Neoplasms; Esophageal adenocarcinoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Melanoma; Gastrointestinal Neoplasms; Somatostatinoma; Adenocarcinoma; Neoplasm Metastasis; Meningioma; Neoplasms; Solid tumours; Ovarian Neoplasms; Rectal Neoplasms; Carcinoma, Renal Cell; Hemangiosarcoma; Carcinoid Tumor; Insulinoma; Carcinoma, Islet Cell; Stomach Neoplasms; Esophageal Neoplasms; Liver Neoplasms; Carcinoma, Transitional Cell; Colonic Neoplasms; Pancreatic Neoplasms; Glioblastoma; Carcinoma, Ovarian Epithelial; Adenoma; Glucagonoma; Carcinoma, Adenoid Cystic; Sarcoma | Details |
| Nintedanib Esylate | BIBF-1120 | Approved | C.H. Boehringer Sohn Ag & Co. Kg | Ofev, Vargatef | United States | Idiopathic Pulmonary Fibrosis | Boehringer Ingelheim Gmbh | 2014-10-15 | Lung Neoplasms; Breast Neoplasms; Prostatic Neoplasms; Adenocarcinoma, Clear Cell; systemic sclerosis-associated interstitial lung disease; Colorectal Neoplasms; Peritoneal Neoplasms; Gliosarcoma; Hepatic Insufficiency; Astrocytoma; Genital Neoplasms, Female; Silicosis; Sarcoma; Carcinoma, Squamous Cell; Fallopian Tube Neoplasms; Appendiceal Neoplasms; Leukemia, Myeloid, Acute; Uterine Neoplasms; Endometrial Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Adenocarcinoma; Carcinoma, Endometrioid; Scleroderma, Systemic; Telangiectasia, Hereditary Hemorrhagic; Solid tumours; Rejection of lung transplantation; Carcinoma, Renal Cell; Radiation Pneumonitis; Esophageal Neoplasms; Carcinoid Tumor; Endometrial Stromal Tumors; Idiopathic Pulmonary Fibrosis; Neoplasms; Ovarian Neoplasms; Glioblastoma; Colonic Neoplasms; Small Cell Lung Carcinoma; Pulmonary Fibrosis; Lung Diseases, Interstitial; Oligodendroglioma; Multiple Myeloma; Mesothelioma; Asbestosis; Neuroendocrine Tumors | Details |
| Vorolanib | X-82; EYP-1901; CM-082 | Approved | Tyrogenex Inc | 伏美纳 | Mainland China | Carcinoma, Renal Cell | Betta Pharmaceuticals Co Ltd | 2023-06-07 | Diabetic macular oedema; Carcinoma, Non-Small-Cell Lung; Melanoma; Macular Degeneration; Carcinoma, Hepatocellular; Diabetic Retinopathy; Choroidal Neovascularization; Adenocarcinoma; Retinal Degeneration; Leukemia, Myeloid, Acute; Eye Diseases; Solid tumours; Wet Macular Degeneration; Retinal Diseases; Small Cell Lung Carcinoma; Neoplasms; Pancreatic Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Carcinoma, Renal Cell; Kidney Neoplasms | Details |
| Tivozanib | Kil-8951; AV-951; KRN-951; ASP-4130; KHK-4951; KHK4951; UNII-172030934T | Approved | Kyowa Hakko Kirin Co Ltd | Fotivda, FOTIVDA | EU | Carcinoma, Renal Cell | Recordati Netherlands BV | 2017-08-24 | Colorectal Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Gastrointestinal Neoplasms; Macular Degeneration; Fallopian Tube Neoplasms; Metastatic breast cancer; Peritoneal Neoplasms; Hepatic Insufficiency; Bile Duct Neoplasms; Biliary Tract Neoplasms; Sarcoma; Breast Neoplasms; Cholangiocarcinoma; Carcinoma, Ovarian Epithelial; Triple Negative Breast Neoplasms; Carcinoma, Renal Cell; Ovarian Neoplasms; Liver Neoplasms; Solid tumours | Details |
| Apatinib Mesylate | YN-968D1 | Approved | Advenchen Laboratories Llc | 艾坦, Aitan | Mainland China | Stomach Neoplasms | Jiangsu shengdi Medicine Co Ltd | 2014-10-17 | Nasopharyngeal Neoplasms; Adenocarcinoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Lung Neoplasms; Fallopian Tube Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Nasopharyngeal Carcinoma; Breast Neoplasms; Carcinoma, Adenoid Cystic; Head and Neck Neoplasms; Triple Negative Breast Neoplasms; Carcinoma, Ovarian Epithelial; Neoplasms; Esthesioneuroblastoma, Olfactory; Esophageal Neoplasms; Stomach Neoplasms; Solid tumours; Ovarian Neoplasms; Liver Neoplasms; Biliary Tract Neoplasms | Details |
| Ripretinib | DCC-2618 | Approved | Deciphera | Qinlock, 擎乐 | United States | Gastrointestinal Stromal Tumors | Deciphera Pharmaceuticals Llc | 2020-05-15 | Neoplasms; Mastocytosis, Systemic; Gastrointestinal Stromal Tumors | Details |
| Ramucirumab | IMC-1121; IMC-1C11; LY-3009806; IMC-1121-B; BLA-125477 | Approved | Dyax Pharma | Cyramza | United States | Gastrointestinal Stromal Tumors | Eli Lilly And Company | 2014-04-21 | Breast Neoplasms; Melanoma; Carcinoma, Hepatocellular; Adenocarcinoma; Carcinoma, Non-Small-Cell Lung; Fallopian Tube Neoplasms; Ureteral Neoplasms; Urologic Neoplasms; Peritoneal Neoplasms; Gastrointestinal Stromal Tumors; Colorectal Neoplasms; Prostatic Neoplasms; Urethral Neoplasms; Liver Neoplasms; Pancreatic Neoplasms; Colonic Neoplasms; Carcinoma, Transitional Cell; Neoplasms; Carcinoma, Renal Cell; Rectal Neoplasms; Esophageal Neoplasms; Carcinoid Tumor; Stomach Neoplasms; Biliary Tract Neoplasms; Ovarian Neoplasms; Solid tumours | Details |
| Sunitinib Malate | PNU-290940AD; PHA-290940AD; SCAI-003; GB-102; PNU-290940; SU-011248-L-malate salt; PHA-290940; SU-010398; SU-11248 | Approved | Pfizer Inc | 索坦, Sutent | United States | Carcinoma, Renal Cell; Gastrointestinal Stromal Tumors | Cppi Cv | 2006-01-26 | Solid tumours; Fibromatosis, Aggressive; Ovarian Neoplasms; Kidney Neoplasms; Leukemia, Myelogenous, Chronic; Head and Neck Neoplasms; Leiomyosarcoma; Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; HIV Infections; Leukemia, Myeloid, Accelerated Phase; Leukemia; Fibrosarcoma; Teratoma; Liver Neoplasms; Ependymoma; Lymphoma, T-Cell, Peripheral; Intestinal Neoplasms; Histiocytoma, Malignant Fibrous; Carcinoma, Renal Cell; Hemangioblastoma; Carcinoma, Islet Cell; Carcinoma; Pheochromocytoma; Stomach Neoplasms; Pelvic Neoplasms; Esophageal Neoplasms; Leukemia, Hairy Cell; Polycythemia Vera; Abdominal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Thoracic Neoplasms; Pancreatic neuroendocrine tumors (pNET); Carcinoma, Ovarian Epithelial; Glioblastoma; Neurofibromatoses; Small Cell Lung Carcinoma; Adenoma, Islet Cell; Carcinoma, Transitional Cell; Pancreatic Neoplasms; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Myelodysplastic Syndromes; Hodgkin Disease; Leukemia, Myelomonocytic, Chronic; Carcinoma, Papil | Details |
| Pazopanib Hydrochloride | GSK-786034; GW-786034B; SB-786034; GW-786034 | Approved | Glaxosmithkline Plc, Novartis Pharma Ag | 维全特, Armala, Votrient, Patorma | United States | Carcinoma, Renal Cell; Sarcoma | Novartis Pharma Ag | 2009-10-19 | Uterine Cervical Diseases; Fallopian Tube Neoplasms; Lung Neoplasms; Uterine Neoplasms; Choriocarcinoma; Lymphoma; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Carcinoma, Mucoepidermoid; Gliosarcoma; Genital Neoplasms, Female; Leukemia, Myeloid, Acute; Peritoneal Neoplasms; Brain Neoplasms; Urethral Neoplasms; Medullary thyroid cancer (MTC); Chondrosarcoma, Extraskeletal Myxoid; Prostatic Neoplasms; Osteosarcoma; Neuroblastoma; Sarcoma; Breast Neoplasms; Carcinoma, Hepatocellular; Uterine Cervical Neoplasms; Breast Neoplasms, Male; Neoplasm Metastasis; von Hippel-Lindau Disease; Thyroid Cancer, Papillary; Gastrointestinal Neoplasms; Paraganglioma; Endodermal Sinus Tumor; Neoplasms, Germ Cell and Embryonal; Melanoma; Gastrinoma; Macular Degeneration; Carcinoma, Non-Small-Cell Lung; Thyroid Neoplasms; Carcinoma, Squamous Cell; Carcinoma, Small Cell; Carcinoma, Embryonal; Germinoma; Glioma; Carcinoma, Neuroendocrine; Epistaxis; Carcinoma, Ovarian Epithelial; Neoplasms; Squamous Cell Carcinoma of Head a | Details |
| Cabozantinib S-malate | XL-184; BMS-907351 | Approved | Exelixis Inc | Cometriq, Cabometyx | United States | Carcinoma, Neuroendocrine; Thyroid Neoplasms | Exelixis Inc | 2012-11-29 | Urethral Neoplasms; Leukemia, Myeloid, Acute; Thyroid Neoplasms; Gliosarcoma; Colorectal Neoplasms; Astrocytoma; Hepatic Insufficiency; Peritoneal Neoplasms; Bile Duct Neoplasms; Sarcoma, Clear Cell; Uterine Neoplasms; Adenocarcinoma, Clear Cell; Sarcoma, Ewing; Carcinoma, Adenosquamous; Breast Neoplasms; Neurofibroma, Plexiform; Osteosarcoma; Prostatic Neoplasms; Sarcoma; Medullary thyroid cancer (MTC); Carcinoma, Hepatocellular; Melanoma; Paraganglioma; Meningioma; Neoplasms, Germ Cell and Embryonal; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Carcinoma, Endometrioid; Thyroid Cancer, Papillary; Brain Neoplasms; Carcinoma, Neuroendocrine; Sarcoma, Alveolar Soft Part; Lymphoma; Fallopian Tube Neoplasms; Brain metastases; Glioma; Endometrial Neoplasms; Carcinoma, Squamous Cell; Carcinoid Tumor; Glioblastoma; Skin Neoplasms; Neoplasms; Carcinoma, Papillary; Hepatoblastoma; Pheochromocytoma; Pain; Rejection of liver transplantation; Carcinoma, Renal Cell; Pancreatic neuroendocrine tumors | Details |
| Fruquintinib | HMPL-013; TAK-113 | Approved | Hutchison Medipharma Ltd | Elunate, FRUZAQLA, Aiyoute, 爱优特 | Mainland China | Colorectal Neoplasms | Hutchison Medipharma Ltd | 2018-09-04 | Small Cell Lung Carcinoma; Carcinoma, Non-Small-Cell Lung; Endometrial Neoplasms; Lung Neoplasms; Hepatic Insufficiency; Colorectal Neoplasms; Breast Neoplasms; Sarcoma; Kidney Diseases; Neoplasms; Solid tumours; Triple Negative Breast Neoplasms; Pancreatic Neoplasms; Colonic Neoplasms; Rectal Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Renal Cell; Biliary Tract Neoplasms | Details |
| Sorafenib Tosylate | NSC-724772; BAY-43-0006; BAY-43-9006; BAY-54-9085 | Approved | Onyx Pharmaceuticals Inc | Nexavar, 多吉美 | United States | Carcinoma, Renal Cell | Bayer Healthcare Pharmaceuticals Inc | 2005-12-01 | Liver Neoplasms; Kidney Neoplasms; Recurrence; Ovarian Neoplasms; Leukemia, Myelogenous, Chronic; Lymphoma, T-Cell, Peripheral; Leukemia, Erythroblastic, Acute; Rhabdomyosarcoma; Lymphoma, B-Cell, Marginal Zone; Fibromatosis, Aggressive; Head and Neck Neoplasms; Solid tumours; Leiomyosarcoma; Leukemia, Myeloid; Carcinoma, Renal Cell; Carcinoma; Vipoma; Esophageal Neoplasms; Hemangiosarcoma; Squamous Cell Carcinoma of Head and Neck; Rectal Neoplasms; Carcinoid Tumor; Histiocytoma, Malignant Fibrous; Carcinoma, Islet Cell; Insulinoma; Stomach Neoplasms; Neoplasms; Kidney Diseases; Small Cell Lung Carcinoma; Carcinoma, Transitional Cell; Carcinoma, Verrucous; Thyroid Carcinoma, Anaplastic; Myelodysplastic Syndromes; Glioblastoma; Pancreatic Neoplasms; Leukemia, Myelomonocytic, Chronic; Carcinoma, Ovarian Epithelial; Leukemia, Myelomonocytic, Acute; Wilms Tumor; Lymphomatoid Granulomatosis; Colonic Neoplasms; Hypertension, Portal; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Large-Cell, Immunoblastic; Multiple Endo | Details |
| Vandetanib | AZD-6474; CH-331; ZD-6474 | Approved | Astrazeneca Plc | Zactima, Caprelsa | United States | Carcinoma, Neuroendocrine; Thyroid Neoplasms | Genzyme Corp | 2011-04-06 | Lung Neoplasms; Neuroblastoma; Medullary thyroid cancer (MTC); Prostatic Neoplasms; Urethral Neoplasms; Peritoneal Neoplasms; Gliosarcoma; Colorectal Neoplasms; Ureteral Neoplasms; Gastrointestinal Stromal Tumors; Diffuse Intrinsic Pontine Glioma; Lymphoproliferative Disorders; Astrocytoma; Thyroid Neoplasms; Pleural Effusion; Carcinoma, Squamous Cell; Lymphoma; Fallopian Tube Neoplasms; Multiple Endocrine Neoplasia Type 2b; Glioma; Carcinoma, Neuroendocrine; Carcinoma, Non-Small-Cell Lung; Paraganglioma; Precancerous Conditions; Neoplasm Metastasis; Adenocarcinoma; von Hippel-Lindau Disease; Carcinoma, Hepatocellular; Anus Neoplasms; Biliary Tract Neoplasms; Solid tumours; Kidney Neoplasms; Intestinal Neoplasms; Ovarian Neoplasms; Pheochromocytoma; Stomach Neoplasms; Esophageal Neoplasms; Carcinoma, Renal Cell; Carcinoma; Squamous Cell Carcinoma of Head and Neck; Rectal Neoplasms; Abdominal Neoplasms; Head and Neck Neoplasms; Small Cell Lung Carcinoma; Carcinoma, Transitional Cell; Pancreatic Neoplasms; Neop | Details |
| Midostaurin | PKC-412; PKC-412A; CGP-41231; CGP-41251 | Approved | Novartis Pharma Ag | Rydapt | United States | Mastocytosis, Systemic; Leukemia, Mast-Cell; Hematologic Neoplasms; Leukemia, Myeloid, Acute | Novartis Pharmaceuticals Corp | 2017-04-28 | Leukemia; Hematologic Neoplasms; Mastocytosis, Systemic; Myelodysplastic Syndromes; Leukemia, Mast-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Hepatic Insufficiency; Leukemia, Myeloid, Acute; Mastocytosis | Details |
| English Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Zanzalintinib | XL-092 | Phase 3 Clinical | Exelixis Inc | Solid tumours; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Transitional Cell; Neoplasms; Prostatic Neoplasms, Castration-Resistant; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| JY-025 | JY-025 | Phase 3 Clinical | Beijing Jingyitaixiang Technology Development Co Ltd, Beijing Eastern Biotech Co Ltd | Liver Neoplasms; Solid tumours; Stomach Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Recombinant anti-VEGFR2 chimeric monoclonaly antibody (GeneScience) | Phase 3 Clinical | Changchun GeneScience Pharmaceuticals Co Ltd | Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Gastrointestinal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| Lucitanib | S-80881; AL-3810; CO-3810; E-3810; S-80881-2 | Phase 3 Clinical | Advenchen Laboratories Nanjing Ltd | Solid tumours; Stomach Neoplasms; Small Cell Lung Carcinoma; Nasopharyngeal Carcinoma; Breast Neoplasms; Colorectal Neoplasms; Genital Neoplasms, Female; Thymus Neoplasms; Lung Neoplasms; Carcinoma, Small Cell; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| Recombinant human anti-VEGFR2 monoclonal antibody (Buchang Pharma) | BC-001; BC001 | Phase 3 Clinical | Shandong Buchang Pharmaceuticals Co Ltd | Solid tumours; Esophageal Neoplasms; Colorectal Neoplasms | Details |
| KC1036 | KC1036; KC-1036 | Phase 3 Clinical | Beijing Konruns Pharmaceutical Co Ltd | Hematologic Neoplasms; Solid tumours; Digestive System Neoplasms; Sarcoma, Ewing; Thymus Neoplasms; Esophageal Squamous Cell Carcinoma; Adenocarcinoma; Neoplasm Metastasis | Details |
| Cediranib | AZD2171; AZD-2171; NSC-732208 | Phase 3 Clinical | Astrazeneca Plc | Solid tumours; Ovarian Neoplasms; Head and Neck Neoplasms; Biliary Tract Neoplasms; Leiomyosarcoma; Medulloblastoma; Leukemia; Ependymoma; Lymphoma, T-Cell, Peripheral; Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Liver Neoplasms; Carcinoma; Rhabdoid Tumor; Leukemia, Hairy Cell; Squamous Cell Carcinoma of Head and Neck; Rectal Neoplasms; Carcinoma, Renal Cell; Stomach Neoplasms; Cystadenocarcinoma, Serous; Spinal Cord Neoplasms; Cystadenoma, Serous; Cystadenocarcinoma, Mucinous; Glioblastoma; Pancreatic Neoplasms; Carcinoma, Verrucous; Neoplasms; Nasopharyngeal Neoplasms; Myelodysplastic Syndromes; Carcinoma, Ovarian Epithelial; Hodgkin Disease; Hypopharyngeal Neoplasms; Colonic Neoplasms; Salivary Gland Neoplasms; Lymphomatoid Granulomatosis; Lymphoma, Large-Cell, Immunoblastic; Lymphoma, Large B-Cell, Diffuse; Small Cell Lung Carcinoma; Leukemia-Lymphoma, Adult T-Cell; Carcinoma, Transitional Cell; Triple Negative Breast Neoplasms; Oligodendroglioma; Uveal melanoma; Adenocarcinoma, Follicular; Neuroen | Details |
| Sitravatinib | MG-91516; MGCD-516; IND-155305; MG-516 | Phase 3 Clinical | Mirati Therapeutics Inc | Breast Neoplasms; Carcinoma, Hepatocellular; Gastrointestinal Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung; Metastatic breast cancer; Mouth Neoplasms; Endometrial Neoplasms; Carcinoma, Squamous Cell; Esophageal Squamous Cell Carcinoma; Lung Neoplasms; Ureteral Neoplasms; Hepatic Insufficiency; Solid tumours; Liposarcoma; Uveal melanoma; Lung Diseases; Carcinoma, Transitional Cell; Neoplasms; Triple Negative Breast Neoplasms; Carcinoma; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Biliary Tract Neoplasms; Kidney Neoplasms | Details |
| Gentuximab | Phase 3 Clinical | Genescience Pharmaceuticals Co Ltd | Adenocarcinoma; Carcinoma, Non-Small-Cell Lung | Details | |
| Axitinib intravitreal implant (Ocular Therapeutix) | OTX-TKI | Phase 3 Clinical | Ocular Therapeutix Inc | Wet Macular Degeneration; Macular Degeneration; Diabetic Retinopathy | Details |
| Pulocimab | AK-109; AK109 | Phase 3 Clinical | Kang Rong Dongfang (Guangdong) Pharmaceutical Co Ltd | Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Adenocarcinoma | Details |
| Ningetinib Tosylate | CT-053-PTSA; CT-053 | Phase 2 Clinical | Hec Pharm Co Ltd | Intestinal Neoplasms; Solid tumours; Carcinoma, Renal Cell; Stomach Neoplasms; Leukemia, Myeloid, Acute; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Simmitinib hydrochloride | SOMCL-15-290 | Phase 2 Clinical | Shanghai Institute Of Materia Medica, Chinese Academy Of Sciences | Solid tumours; Neoplasms; Esophageal Squamous Cell Carcinoma | Details |
| AUR-109 | ODM-203; AUR-109 | Phase 2 Clinical | Orion Corp | Ovarian Neoplasms; Liver Neoplasms; Solid tumours; Carcinoma, Renal Cell; Urinary Bladder Neoplasms; Pulmonary Fibrosis; Breast Neoplasms; Lung Neoplasms | Details |
| VEGFR2 peptide vaccine (VAXIMM) | VXM-01 | Phase 2 Clinical | Merck Serono | Glioblastoma; Pancreatic Neoplasms; Colorectal Neoplasms | Details |
| Telatinib | EOC-315; BAY-57-9352 | Phase 2 Clinical | Bayer AG | Solid tumours; Stomach Neoplasms | Details |
| Brivanib Alaninate | ZL-2301; BMS-540215; BMS-582664 | Phase 2 Clinical | Bristol-Myers Squibb Company | Carcinoma, Squamous Cell; Carcinoma, Endometrioid; Adenocarcinoma; Uterine Cervical Neoplasms; Neoplasm Metastasis; Gastrointestinal Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Endometrial Neoplasms; Liver Neoplasms; Colorectal Neoplasms; Sarcoma; Neoplasms; Pancreatic Neoplasms; Carcinoma, Transitional Cell; Carcinoma, Renal Cell; Rectal Neoplasms; Solid tumours | Details |
| Derazantinib | ARQ-087.2HCl; AQ-14741087; ARQ-087; BAL-087 | Phase 2 Clinical | Arqule Inc | Biliary Tract Neoplasms; Solid tumours; Stomach Neoplasms; Carcinoma, Transitional Cell; Cholangiocarcinoma; Urogenital Neoplasms; Carcinoma, Hepatocellular | Details |
| Glesatinib | MGCD-265; MG-90265X; 7Q29OXD98N; MG-90265H9; MG-90265gly; MG-90265 | Phase 2 Clinical | Mirati Therapeutics Inc | Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Olinvacimab | SSS-23; TTAC-0001 | Phase 2 Clinical | Pharmabcine | Neoplasms; Glioblastoma; Triple Negative Breast Neoplasms; Neoplasm Metastasis | Details |
| BR-55 | BR-55; BR55 CEUS | Phase 2 Clinical | Sonus Pharmaceuticals Ltd | Ovarian Neoplasms; Neoplasms; Contrast agents; Diagnostic agents; Inflammation | Details |
| Axitinib Implant(Aerie) | AR-14034 SR; AR-14034 | Phase 2 Clinical | Aerie Pharmaceuticals Inc | Macular Degeneration | Details |
| Sorafenib Tosylate/Comekibart | MG-D-1609 | Phase 2 Clinical | Metagone Biotech Inc | Solid tumours | Details |
| VEGFR-1/2 peptide vaccine (Keio University) | Phase 2 Clinical | Keio University | Neurofibromatoses; Brain Neoplasms; Glioma | Details | |
| Gersizangitide | AXT-107 | Phase 2 Clinical | AsclepiX Therapeutics Inc | Macular Edema; Diabetic macular oedema; Macular Degeneration | Details |
| KDR2-2 | Phase 2 Clinical | Guangzhou Huiborui Biomedical Technology Co Ltd | Neovascularization, Pathologic; Glaucoma, Neovascular; Corneal Neovascularization | Details | |
| KHK-4951 | Phase 2 Clinical | Kyowa Kirin | Macular Edema; Wet Macular Degeneration; Diabetic macular oedema; Macular Degeneration | Details | |
| PAN-90806 | CP-632; OSI-632; CP-547632; PAN-90806 | Phase 2 Clinical | Pfizer Inc, Osi Pharmaceutical | Ovarian Neoplasms; Abdominal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Lung Neoplasms; Macular Degeneration; Diabetic Retinopathy | Details |
| CEP-11981 | ESK-981; CEP-11981; SSR-106462; BOL-303213X | Phase 2 Clinical | Sanofi | Adenoma, Islet Cell; Pancreatic Neoplasms; Neoplasms; Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms; Carcinoma, Adenosquamous; Carcinoma, Neuroendocrine; Gastrointestinal Neoplasms | Details |
| Axitinib injectable suspension (Clearside Biomedical) | CLS-1002; CLS-011-A; CLS011A; CLS-AX | Phase 2 Clinical | Clearside Biomedical Inc | Macular Degeneration | Details |
| MSB-0254 | MSB-0254 | Phase 1 Clinical | Mabspace Biomedicine (Suzhou) Co Ltd | Solid tumours | Details |
| EDP317 | ACTB-1003; EOC-317; EDP-317 | Phase 1 Clinical | Bayer AG, Act Biotech Inc | Biliary Tract Neoplasms; Solid tumours; Stomach Neoplasms; Urinary Bladder Neoplasms; Breast Neoplasms | Details |
| Kanitinib | CX-1003 | Phase 1 Clinical | Beijing Konruns Pharmaceutical Co Ltd, Guangzhou Yingsheng Bio Technology Co Ltd | Solid tumours | Details |
| VEGFR2 peptide vaccine (Tokyo University) | Phase 1 Clinical | University Of Tokyo | Pancreatic Neoplasms | Details | |
| Ramucirumab biosimilar (Henlius) | HLX-12 | Phase 1 Clinical | Shanghai Henlius Biotech Inc | Stomach Neoplasms; Esophageal Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| AVI-3207 | AVI-3207 | Phase 1 Clinical | Avixgen | Macular Degeneration | Details |
| Pamufetinib | TAS-115 | Phase 1 Clinical | Taiho Pharmaceutical Co Ltd, Otsuka Pharmaceutical Co Ltd | Neoplasms | Details |
| Ramucirumab biosimilar (Sichuan Kelunbotai Biopharmaceutical) | Phase 1 Clinical | Sichuan Kelun Pharmaceutical Co Ltd | Solid tumours; Stomach Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| Tafetinib Malate | SIM-0702; SIM-1005; SIM-010603 | Phase 1 Clinical | Jiangsu Simcere Pharmaceutical Co Ltd, Nanjing Yoko Biomedical Co Ltd, Jilin Boda Pharmaceutical Co Ltd | Neoplasms | Details |
| QBH-196 | QBH-196 | Phase 1 Clinical | Shenyang Pharmaceutical University | Solid tumours; Stomach Neoplasms | Details |
| ETN-101 | ETN101; ETN-101; MBP-11901 | Phase 1 Clinical | Etnova Therapeutics Corp | Liver Neoplasms; Carcinoma, Hepatocellular | Details |
| RA1115-B1 | RA1115-B1 | Phase 1 Clinical | Suzhou Raymon Pharmaceuticals Co Ltd | Wet Macular Degeneration; Macular Degeneration | Details |
| PZ-1 | PZ-1 | Phase 1 Clinical | Chongqing University Of Arts And Sciences | Solid tumours | Details |
| SYHA-1817 | SYHA-1817 | Phase 1 Clinical | CSPC Pharmaceutical Group Ltd | Biliary Tract Neoplasms; Stomach Neoplasms; Carcinoma, Squamous Cell | Details |
| Ramucirumab biosimilar (CTTQ) | Phase 1 Clinical | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | Stomach Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| LMV-12(HE003) | LMV-12(HE003) | Phase 1 Clinical | Nanchang Hongyi Technology Co Ltd | Solid tumours | Details |
| Metatinib Trometamol | BMS-817378; SIM-817378 | Phase 1 Clinical | Bristol-Myers Squibb Company | Solid tumours; Liver Neoplasms; Stomach Neoplasms; Colorectal Neoplasms | Details |
| NP-01 (Shijiazhuang No.4 Pharmaceutical/Nanjing Nadingfei Medical Technology) | NP-01 | Phase 1 Clinical | Shijiazhuang No 4 Pharmaceutical Co Ltd, Nanjing Nadingfei Pharmaceutical Technology Co Ltd | Solid tumours; Liver Neoplasms; Stomach Neoplasms; Lung Neoplasms | Details |
| FNX-006 | FNX-006 | Phase 1 Clinical | Sichuan University, Chengdu Fannuoxi Biomedical Technology Co Ltd | Triple Negative Breast Neoplasms; Melanoma | Details |
| Zanzalintinib | XL-092 | Phase 3 Clinical | Exelixis Inc | Solid tumours; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Transitional Cell; Neoplasms; Prostatic Neoplasms, Castration-Resistant; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| JY-025 | JY-025 | Phase 3 Clinical | Beijing Jingyitaixiang Technology Development Co Ltd, Beijing Eastern Biotech Co Ltd | Liver Neoplasms; Solid tumours; Stomach Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Recombinant anti-VEGFR2 chimeric monoclonaly antibody (GeneScience) | Phase 3 Clinical | Changchun GeneScience Pharmaceuticals Co Ltd | Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Gastrointestinal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| Lucitanib | S-80881; AL-3810; CO-3810; E-3810; S-80881-2 | Phase 3 Clinical | Advenchen Laboratories Nanjing Ltd | Solid tumours; Stomach Neoplasms; Small Cell Lung Carcinoma; Nasopharyngeal Carcinoma; Breast Neoplasms; Colorectal Neoplasms; Genital Neoplasms, Female; Thymus Neoplasms; Lung Neoplasms; Carcinoma, Small Cell; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| Recombinant human anti-VEGFR2 monoclonal antibody (Buchang Pharma) | BC-001; BC001 | Phase 3 Clinical | Shandong Buchang Pharmaceuticals Co Ltd | Solid tumours; Esophageal Neoplasms; Colorectal Neoplasms | Details |
| KC1036 | KC1036; KC-1036 | Phase 3 Clinical | Beijing Konruns Pharmaceutical Co Ltd | Hematologic Neoplasms; Solid tumours; Digestive System Neoplasms; Sarcoma, Ewing; Thymus Neoplasms; Esophageal Squamous Cell Carcinoma; Adenocarcinoma; Neoplasm Metastasis | Details |
| Cediranib | AZD2171; AZD-2171; NSC-732208 | Phase 3 Clinical | Astrazeneca Plc | Solid tumours; Ovarian Neoplasms; Head and Neck Neoplasms; Biliary Tract Neoplasms; Leiomyosarcoma; Medulloblastoma; Leukemia; Ependymoma; Lymphoma, T-Cell, Peripheral; Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Liver Neoplasms; Carcinoma; Rhabdoid Tumor; Leukemia, Hairy Cell; Squamous Cell Carcinoma of Head and Neck; Rectal Neoplasms; Carcinoma, Renal Cell; Stomach Neoplasms; Cystadenocarcinoma, Serous; Spinal Cord Neoplasms; Cystadenoma, Serous; Cystadenocarcinoma, Mucinous; Glioblastoma; Pancreatic Neoplasms; Carcinoma, Verrucous; Neoplasms; Nasopharyngeal Neoplasms; Myelodysplastic Syndromes; Carcinoma, Ovarian Epithelial; Hodgkin Disease; Hypopharyngeal Neoplasms; Colonic Neoplasms; Salivary Gland Neoplasms; Lymphomatoid Granulomatosis; Lymphoma, Large-Cell, Immunoblastic; Lymphoma, Large B-Cell, Diffuse; Small Cell Lung Carcinoma; Leukemia-Lymphoma, Adult T-Cell; Carcinoma, Transitional Cell; Triple Negative Breast Neoplasms; Oligodendroglioma; Uveal melanoma; Adenocarcinoma, Follicular; Neuroen | Details |
| Sitravatinib | MG-91516; MGCD-516; IND-155305; MG-516 | Phase 3 Clinical | Mirati Therapeutics Inc | Breast Neoplasms; Carcinoma, Hepatocellular; Gastrointestinal Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung; Metastatic breast cancer; Mouth Neoplasms; Endometrial Neoplasms; Carcinoma, Squamous Cell; Esophageal Squamous Cell Carcinoma; Lung Neoplasms; Ureteral Neoplasms; Hepatic Insufficiency; Solid tumours; Liposarcoma; Uveal melanoma; Lung Diseases; Carcinoma, Transitional Cell; Neoplasms; Triple Negative Breast Neoplasms; Carcinoma; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Biliary Tract Neoplasms; Kidney Neoplasms | Details |
| Gentuximab | Phase 3 Clinical | Genescience Pharmaceuticals Co Ltd | Adenocarcinoma; Carcinoma, Non-Small-Cell Lung | Details | |
| Axitinib intravitreal implant (Ocular Therapeutix) | OTX-TKI | Phase 3 Clinical | Ocular Therapeutix Inc | Wet Macular Degeneration; Macular Degeneration; Diabetic Retinopathy | Details |
| Pulocimab | AK-109; AK109 | Phase 3 Clinical | Kang Rong Dongfang (Guangdong) Pharmaceutical Co Ltd | Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Adenocarcinoma | Details |
| Ningetinib Tosylate | CT-053-PTSA; CT-053 | Phase 2 Clinical | Hec Pharm Co Ltd | Intestinal Neoplasms; Solid tumours; Carcinoma, Renal Cell; Stomach Neoplasms; Leukemia, Myeloid, Acute; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Simmitinib hydrochloride | SOMCL-15-290 | Phase 2 Clinical | Shanghai Institute Of Materia Medica, Chinese Academy Of Sciences | Solid tumours; Neoplasms; Esophageal Squamous Cell Carcinoma | Details |
| AUR-109 | ODM-203; AUR-109 | Phase 2 Clinical | Orion Corp | Ovarian Neoplasms; Liver Neoplasms; Solid tumours; Carcinoma, Renal Cell; Urinary Bladder Neoplasms; Pulmonary Fibrosis; Breast Neoplasms; Lung Neoplasms | Details |
| VEGFR2 peptide vaccine (VAXIMM) | VXM-01 | Phase 2 Clinical | Merck Serono | Glioblastoma; Pancreatic Neoplasms; Colorectal Neoplasms | Details |
| Telatinib | EOC-315; BAY-57-9352 | Phase 2 Clinical | Bayer AG | Solid tumours; Stomach Neoplasms | Details |
| Brivanib Alaninate | ZL-2301; BMS-540215; BMS-582664 | Phase 2 Clinical | Bristol-Myers Squibb Company | Carcinoma, Squamous Cell; Carcinoma, Endometrioid; Adenocarcinoma; Uterine Cervical Neoplasms; Neoplasm Metastasis; Gastrointestinal Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Endometrial Neoplasms; Liver Neoplasms; Colorectal Neoplasms; Sarcoma; Neoplasms; Pancreatic Neoplasms; Carcinoma, Transitional Cell; Carcinoma, Renal Cell; Rectal Neoplasms; Solid tumours | Details |
| Derazantinib | ARQ-087.2HCl; AQ-14741087; ARQ-087; BAL-087 | Phase 2 Clinical | Arqule Inc | Biliary Tract Neoplasms; Solid tumours; Stomach Neoplasms; Carcinoma, Transitional Cell; Cholangiocarcinoma; Urogenital Neoplasms; Carcinoma, Hepatocellular | Details |
| Glesatinib | MGCD-265; MG-90265X; 7Q29OXD98N; MG-90265H9; MG-90265gly; MG-90265 | Phase 2 Clinical | Mirati Therapeutics Inc | Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Olinvacimab | SSS-23; TTAC-0001 | Phase 2 Clinical | Pharmabcine | Neoplasms; Glioblastoma; Triple Negative Breast Neoplasms; Neoplasm Metastasis | Details |
| BR-55 | BR-55; BR55 CEUS | Phase 2 Clinical | Sonus Pharmaceuticals Ltd | Ovarian Neoplasms; Neoplasms; Contrast agents; Diagnostic agents; Inflammation | Details |
| Axitinib Implant(Aerie) | AR-14034 SR; AR-14034 | Phase 2 Clinical | Aerie Pharmaceuticals Inc | Macular Degeneration | Details |
| Sorafenib Tosylate/Comekibart | MG-D-1609 | Phase 2 Clinical | Metagone Biotech Inc | Solid tumours | Details |
| VEGFR-1/2 peptide vaccine (Keio University) | Phase 2 Clinical | Keio University | Neurofibromatoses; Brain Neoplasms; Glioma | Details | |
| Gersizangitide | AXT-107 | Phase 2 Clinical | AsclepiX Therapeutics Inc | Macular Edema; Diabetic macular oedema; Macular Degeneration | Details |
| KDR2-2 | Phase 2 Clinical | Guangzhou Huiborui Biomedical Technology Co Ltd | Neovascularization, Pathologic; Glaucoma, Neovascular; Corneal Neovascularization | Details | |
| KHK-4951 | Phase 2 Clinical | Kyowa Kirin | Macular Edema; Wet Macular Degeneration; Diabetic macular oedema; Macular Degeneration | Details | |
| PAN-90806 | CP-632; OSI-632; CP-547632; PAN-90806 | Phase 2 Clinical | Pfizer Inc, Osi Pharmaceutical | Ovarian Neoplasms; Abdominal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Lung Neoplasms; Macular Degeneration; Diabetic Retinopathy | Details |
| CEP-11981 | ESK-981; CEP-11981; SSR-106462; BOL-303213X | Phase 2 Clinical | Sanofi | Adenoma, Islet Cell; Pancreatic Neoplasms; Neoplasms; Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms; Carcinoma, Adenosquamous; Carcinoma, Neuroendocrine; Gastrointestinal Neoplasms | Details |
| Axitinib injectable suspension (Clearside Biomedical) | CLS-1002; CLS-011-A; CLS011A; CLS-AX | Phase 2 Clinical | Clearside Biomedical Inc | Macular Degeneration | Details |
| MSB-0254 | MSB-0254 | Phase 1 Clinical | Mabspace Biomedicine (Suzhou) Co Ltd | Solid tumours | Details |
| EDP317 | ACTB-1003; EOC-317; EDP-317 | Phase 1 Clinical | Bayer AG, Act Biotech Inc | Biliary Tract Neoplasms; Solid tumours; Stomach Neoplasms; Urinary Bladder Neoplasms; Breast Neoplasms | Details |
| Kanitinib | CX-1003 | Phase 1 Clinical | Beijing Konruns Pharmaceutical Co Ltd, Guangzhou Yingsheng Bio Technology Co Ltd | Solid tumours | Details |
| VEGFR2 peptide vaccine (Tokyo University) | Phase 1 Clinical | University Of Tokyo | Pancreatic Neoplasms | Details | |
| Ramucirumab biosimilar (Henlius) | HLX-12 | Phase 1 Clinical | Shanghai Henlius Biotech Inc | Stomach Neoplasms; Esophageal Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| AVI-3207 | AVI-3207 | Phase 1 Clinical | Avixgen | Macular Degeneration | Details |
| Pamufetinib | TAS-115 | Phase 1 Clinical | Taiho Pharmaceutical Co Ltd, Otsuka Pharmaceutical Co Ltd | Neoplasms | Details |
| Ramucirumab biosimilar (Sichuan Kelunbotai Biopharmaceutical) | Phase 1 Clinical | Sichuan Kelun Pharmaceutical Co Ltd | Solid tumours; Stomach Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| Tafetinib Malate | SIM-0702; SIM-1005; SIM-010603 | Phase 1 Clinical | Jiangsu Simcere Pharmaceutical Co Ltd, Nanjing Yoko Biomedical Co Ltd, Jilin Boda Pharmaceutical Co Ltd | Neoplasms | Details |
| QBH-196 | QBH-196 | Phase 1 Clinical | Shenyang Pharmaceutical University | Solid tumours; Stomach Neoplasms | Details |
| ETN-101 | ETN101; ETN-101; MBP-11901 | Phase 1 Clinical | Etnova Therapeutics Corp | Liver Neoplasms; Carcinoma, Hepatocellular | Details |
| RA1115-B1 | RA1115-B1 | Phase 1 Clinical | Suzhou Raymon Pharmaceuticals Co Ltd | Wet Macular Degeneration; Macular Degeneration | Details |
| PZ-1 | PZ-1 | Phase 1 Clinical | Chongqing University Of Arts And Sciences | Solid tumours | Details |
| SYHA-1817 | SYHA-1817 | Phase 1 Clinical | CSPC Pharmaceutical Group Ltd | Biliary Tract Neoplasms; Stomach Neoplasms; Carcinoma, Squamous Cell | Details |
| Ramucirumab biosimilar (CTTQ) | Phase 1 Clinical | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | Stomach Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| LMV-12(HE003) | LMV-12(HE003) | Phase 1 Clinical | Nanchang Hongyi Technology Co Ltd | Solid tumours | Details |
| Metatinib Trometamol | BMS-817378; SIM-817378 | Phase 1 Clinical | Bristol-Myers Squibb Company | Solid tumours; Liver Neoplasms; Stomach Neoplasms; Colorectal Neoplasms | Details |
| NP-01 (Shijiazhuang No.4 Pharmaceutical/Nanjing Nadingfei Medical Technology) | NP-01 | Phase 1 Clinical | Shijiazhuang No 4 Pharmaceutical Co Ltd, Nanjing Nadingfei Pharmaceutical Technology Co Ltd | Solid tumours; Liver Neoplasms; Stomach Neoplasms; Lung Neoplasms | Details |
| FNX-006 | FNX-006 | Phase 1 Clinical | Sichuan University, Chengdu Fannuoxi Biomedical Technology Co Ltd | Triple Negative Breast Neoplasms; Melanoma | Details |
This web search service is supported by Google Inc.








