
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
As in many other viruses, the surface glycoprotein responsible for viral entry in SARS-CoV-2 is trimeric in form. ACROBiosystems, a provider of full-length SARS-CoV-2 spike proteins for research and development purposes, employs SEC-MALS to ensure that the material it provides consists of the native trimeric form for optimal use in developing inhibitors, diagnostics and vaccines. We asked Mike Chen, CEO of ACROBiosystems, to provide more details, which he kindly agreed to do.
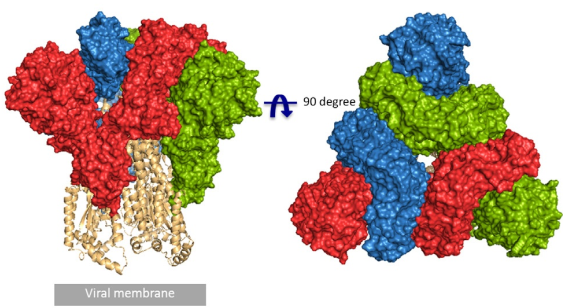
Fig.1 Front and top view of the trimeric coronavirus spike protein ectodomain obtained by cryo-electron microscopy analysis. Three S1 protomers (surface presentation) are colored in red, blue, and green. The S2 trimer (cartoon presentation) is colored in light orange (RCSB PDB structure 6VYB).
Q: Please describe the products and services your company provides.
A: ACROBiosystems is a leading manufacturer of recombinant proteins and other critical reagents to support the development of target therapeutics. The company employs an application-oriented development strategy, with a particular focus on product design, quality control and solution-based support. Our products and services enable anyone in the field of drug development to have a more intuitive and streamlined process.
ACROBiosystems' catalog includes a comprehensive list of disease-associated biomarkers and drug targets from humans to other common species. All of our products are produced with high quality and batch-to-batch consistency to satisfy the rigorous standards of pharmaceutical research and development.
ACROBiosystems continues to grow and adapt to bring more value to our clients by providing additional technical resources and custom services, promoting training and communication opportunities, initiating collaborations in the bio-industry community, and facilitating transactions in the dynamic global and niche markets.
Q: Recently you have been offering both full-length spike protein trimers of the 2019 novel coronavirus, and an inhibitor screening kit. Please tell us about those. How do you foresee customers using them, and what is unique about them?
A: An article published in Science [Wrapp et al, Science, 2020 Mar 13;367(6483):1260-1263. http://doi.org/10.1126/science.abb2507] determined a 3.5-angstrom-resolution cryo-electron microscopy structure of the SARS-CoV-2 S trimer in the prefusion conformation. To facilitate medical countermeasure development, we developed the full-length S [spike – WTC] protein with a trimer structure. The trimer proteins are more suitable for immunization and antibody screening. The predominant state of the trimer has one of the three receptor-binding domains (RBDs) rotated up in a receptor-accessible conformation. The authors also show biophysical and structural evidence that the SARS-CoV-2 S binds ACE2 with higher affinity than SARS-CoV S.
Fig.2 The principle of SARS-CoV-2 inhibitor screening kit.
Furthermore, there is no effective treatment for COVID-19 in the market to date, so it is urgent to develop SARS-CoV-2 inhibitors, for example therapeutic antibodies and small molecular compounds, as well as vaccines against COVID-19. This is why ACROBiosystems developed the SARS-CoV-2 inhibitor screening kit. By providing proteins pre-labeled with biotin, we eliminate the time-consuming labeling process and greatly simplify experimental procedures. This inhibitor screening ELISA kit is designed to facilitate the identification and characterization of SARS-CoV-2 inhibitors. We supply the S protein receptor binding domain (RBD) verified by MALS as well.
Q: You emphasize that the trimer spike protein structure is verified by SEC-MALS. Can you tell us a little about SEC-MALS, and why that is so important to your product validation?
A: Multi-angle light scattering (MALS) is a technique which determines the molecular weight of proteins based on the relationship between the MW, concentration and the intensity of scattered light. This method does not depend on the elution volume, nor does it require protein standards for calibration.
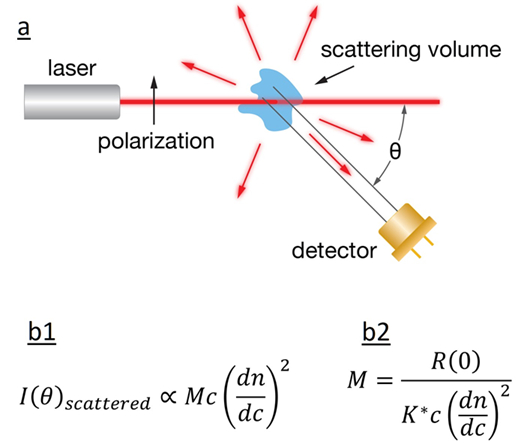
Fig.3 a) Schematic diagram of light scattering; b1) The intensity of the scattered light is related to the product of molar mass, concentration, and square of the refractive index increment; b2) calculation of the sample’s MW.
The correct folding and conformation of recombinant proteins is very important for immunization, antibody screening and identification, functional verification of antibody drug candidates, antibody drug quality control and clinical sample analysis. The oligomeric state of proteins in solution will affect the structure and exposed active sites. The protein will only activate signal pathways and physiological functions effectively when the quaternary structure of the protein is correct.
However, the protein’s native conformation may not correspond to globular protein column calibration standards, especially for glycoproteins. Attempting to determine molecular weight of such proteins against a column calibration curve invariably gives incorrect results. Since MALS does not rely on retention time to calculate the MW, the result is more accurate and reliable than the traditional HPLC-SEC analysis in which the MW is calculated according to the elution time.
We used SEC-MALS to determine the MW of three commercially available antibody drugs including OKT3, Herceptin, and Ipilimumab. As shown in Figure 3, the molecular weights of these three antibodies are actually very similar, but the retention times of the three antibodies are quite different. By column calibration, the OKT3 antibody was estimated at 212.5 kDa, and Ipilimumab was only 66.3 kDa, both of which are far from the true molecular weight. On the other hand, the MW of the three antibodies determined by SEC-MALS is very close to their theoretical (sequence) MW as shown in Figure 4.
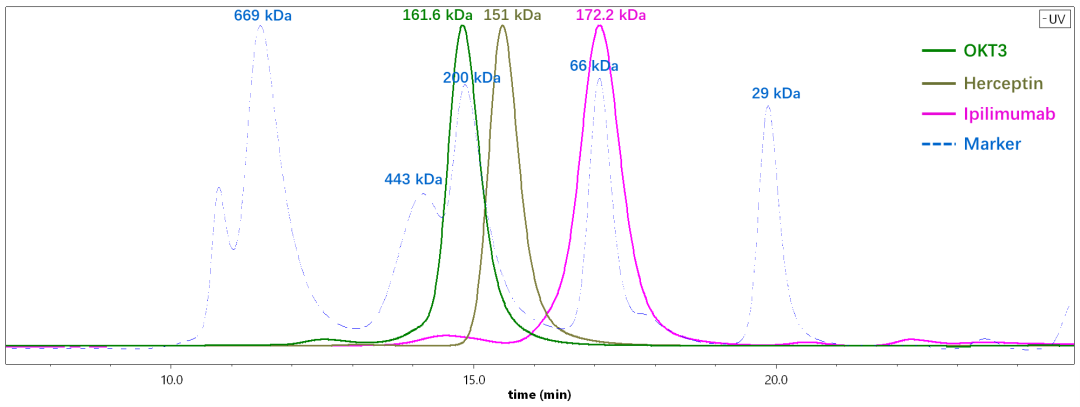
Fig.4 The molecular weights of three different antibodies determined by SEC-MALS are very close to the sequence weights. A) OKT3, b) Herceptin, c) Ipilimumab.
Q: Do customers recognize the value in the SEC-MALS validation of the oligomeric state of the SARS-CoV-2 spike protein?
A: Despite the inherent value of SEC-MALS for determining the oligomeric state of proteins in solution, we suppose most customers don’t fully understand the advantage of this method. We intend to share more data with our customers to show that accurate determination of MW by SEC-MALS is very important for biopolymer analysis.
Click picture to the right to see more MALS-verified SARS-CoV-2(COVID-19) related proteins.
SOURUCE: SEC-MALS in the service of 2019-nCoV therapeutics——Wyatt Technology Corporation.
This web search service is supported by Google Inc.








