 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
> Insights > Inspiring target DLL3: For variety of lung cancer therapeutic methods development The Notch signaling pathway is a highly conserved intercellular signaling pathway involving multiple processes of growth and development, such as differentiation of pluripotent progenitor, apoptosis, cell proliferation, and formation of cell boundaries.
Delta‐like canonical Notch ligand 3 (DLL3) is a member of the Notch receptor ligand family and plays a crucial role in Notch signaling. It is determined that DLL3 is highly expressed in small cell lung cancer (SCLC) and other neuroendocrine tumors, however, the expression of DLL3 is rare in normal tissues.
Therefore, it has aroused people's interest as a new target for cancer treatment. Many more researchers are looking for ways to target DLL-3 for the precision treatment of lung cancer1.
Human DLL3 is a single transmembrane protein that attaches to the cell surface, consisting of 619 amino acids. The complete structure consists of a DSL domain, an intracellular domain, and six epidermal growth factor-like domains.
Different from other Notch ligands, DLL3 is commonly expressed in the Golgi apparatus and appears on the cell surface when overexpressed. It has been found that DLL3 does not bind to the Notch receptor, instead, It is considered as a cell-autonomous inhibitor of Notch signaling2,3.
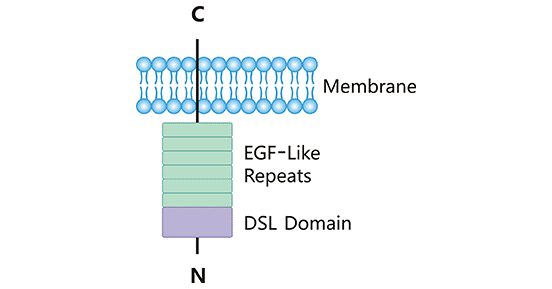
Figure 1. DLL3 structure
>>DLL3 and lung cancer
The latest studies have found that DLL3 is highly expressed on the surface of small cell lung cancer (SCLC) and large cell neuroendocrine carcinoma (LCNEC). Currently, the ADC drug which targets DLL3, ROVA-T, is a promising targeted therapy drug that has shown an effect in SCLC and LCLNEC treatment. Inhibiting the expression of DLL3 can suppress the growth of the two kinds of cells above and induce apoptosis. In addition, DLL3 was found to be a direct downstream target for ASCL1, a transcription factor associated with the development of pulmonary neuroendocrine cells, which suggests that DLL3 is associated with neuroendocrine tumorigenesis, especially in lung cancer-related neuroendocrine tumors 4,5,6.
However, the development of ROVA-T was suspended due to the short overall survival (OS) compared to Topotecan as the positive control 7,8,9. Clinical data show that DLL3 is overexpressed in more than 80% of small cell lung cancer patients. The high expression of DLL3 in SCLC was negatively correlated with the survival time of patients.
>> DLL3 and liver cancer
Epigenetic modifications such as abnormal DNA methylation and histone acetylation in hepatocellular carcinoma (HCC) cells can result in the silencing of DLL3 gene expression. Histone deacetylase inhibitors allow DLL to be expression in HCC, thereby inhibiting the growth of HCC cells and inducing apoptosis.
In addition, the hepatitis B virus protein HBx can also cause epigenetic modifications and inhibit the expression of DLL3 in hepatitis B virus (HBV)-associated HCC. Thus, although it is a therapeutic target due to its high expression in some cancers, DLL3 expression may show different trends in different malignancies11,12.
In recent years, a variety of therapeutic methods targeting DLL3 have been newly developed and advanced to clinical trials with verified therapeutic effects, such as bispecific antibodies, monoclonal antibodies, antibody-coupled drugs (ADCs), cell therapy, and bispecific T-cell conjugates (BiTE).
>> Near-infrared immunotherapy
This technique uses antibody photosensitizer conjugates that use near-infrared light to irradiate and specifically damage cancer cells. Cells incubated with ROVA-IR700, where THE ROVA-T antibody-coupled IR700 photosensitizer is significantly dissolved under near-infrared light irradiation. In addition, significant xenograft shrinkage was observed in mice treated with ROVA-IR700M13.
>> Bispecific T-cell conjugates
Bispecific T‐cell engager (BiTE) is a novel immunotherapy method that redirects a patient's T-cells to kill tumor cells. The AMG 757, developed by Amgen, is optimized to extend the biTE molecular half-life, activating T cells to target tumors expressing DLL3. AMG 757 has been verified d to be effective against SCLC cell lines in vitro; in mouse models. AMG 757 significantly resolves patient-derived xenografts and in situ SCLC tumors by activating T cells. It suggests that AMG 757 is expected to be a novel SCLC immunotherapy drug targeting DLL3. The AMG 757 Clinical Phase I study (NCT03319940) is under way for SCLC patients 14.
>> CAR-T cell therapy
Chimeric antigen receptor T cell therapy involves genetically modifying a patient's own T cells to direct the patient's T-cells for expressing chimeric receptors for tumor antigens. Then reinjects these cells into the patient to attack and kill the target cells. AMG 119 is an adoptive cell therapy that modifies a patient's autologous T cells to express a DLL3 transmembrane chimeric receptor (CAR) and attacks DLL3-positive cells. In vitro assays confirmed that AMG 119 CAR-T cell therapy can reduce SCLC cells expressing DLL3, and in vivo validation found that AMG 119 can inhibit SCLC xenograft tumor growth. Currently, a Phase I clinical study (NCT03392064) is in progress that evaluates the safety, tolerability, and efficacy of AMG 119 for SCLC therapy15.
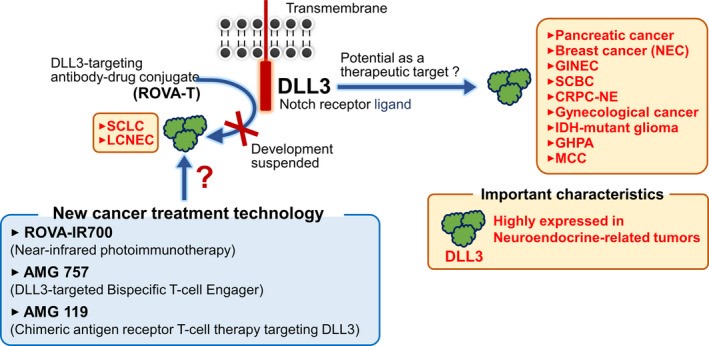
Figure 2. Expression and therapeutic potential of DLL3 in malignant tumors
Click on the image to view product details
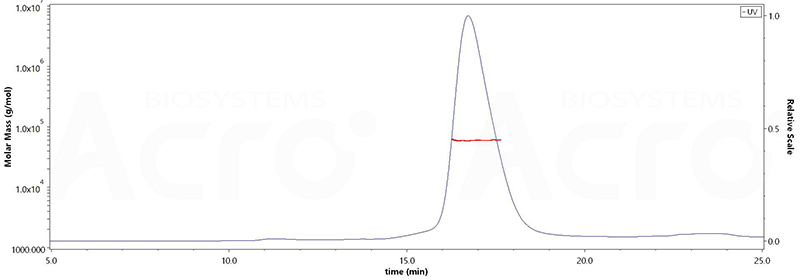
The purity of Human DLL3, His Tag(Cat.No. DL3-H52H4) is more than 95% and the molecular weight of this protein is around 55-75 kDa verified by SEC-MALS.
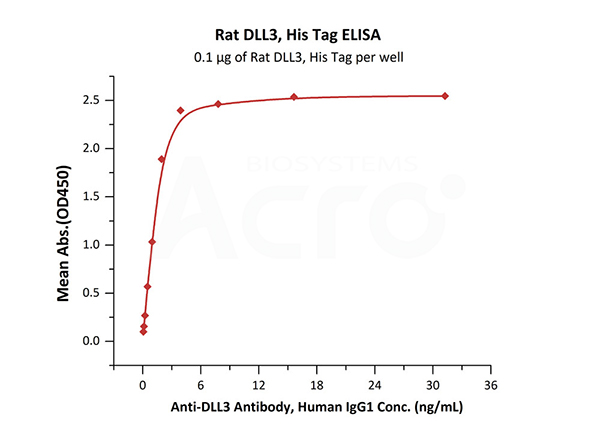
Immobilized Rat DLL3, His Tag (Cat. No. DL3-R52H3) at 1 μg/mL (100 μL/well) can bind Anti-DLL3 Antibody, Human IgG1 with a linear range of 0.1-4 ng/mL (QC tested).
1. Matsuo K, Taniguchi K, Hamamoto H, Inomata Y, Komura K, Tanaka T, et al. Delta-like canonical Notch ligand 3 as a potential therapeutic target in malignancies: A brief overview. Cancer Sci. 2021;112(8):2984-92.
2. Steinbuck, Martin Peter, and Susan Winandy. "A review of notch processing with new insights into ligand-independent notch signaling in T-cells." Frontiers in immunology 9 (2018): 1230.
3. Hu, Bingxin, et al. "Over-expression of human Notch ligand Delta-like 3 promotes proliferation of human gastric cancer cells in vitro." Nan Fang yi ke da xue xue bao= Journal of Southern Medical University 38.1 (2018): 14-19.
4. Augustyn A, Borromeo M, Wang T, Fujimoto J, Shao C, Dospoy PD, et al. ASCL1 is a lineage oncogene providing therapeutic targets for high-grade neuroendocrine lung cancers. Proc Natl Acad Sci U S A. 2014;111(41):14788-93.
5. Geffers I, Serth K, Chapman G, Jaekel R, Schuster-Gossler K, Cordes R, et al. Divergent functions and distinct localization of the Notch ligands DLL1 and DLL3 in vivo. J Cell Biol. 2007;178(3):465-76.
6. Chapman G, Sparrow DB, Kremmer E, Dunwoodie SL. Notch inhibition by the ligand DELTA-LIKE 3 defines the mechanism of abnormal vertebral segmentation in spondylocostal dysostosis. Hum Mol Genet. 2011;20(5):905-16.
7. Rudin CM, Pietanza MC, Bauer TM, Ready N, Morgensztern D, Glisson BS, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2017;18(1):42-51.
8. Saunders LR, Bankovich AJ, Anderson WC, Aujay MA, Bheddah S, Black K, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med. 2015;7(302):302ra136.
9. Matsuo K, Taniguchi K, Hamamoto H, Ito Y, Futaki S, Inomata Y, et al. Delta-like 3 localizes to neuroendocrine cells and plays a pivotal role in gastrointestinal neuroendocrine malignancy. Cancer Sci. 2019;110(10):3122-31.
10. Bauer, Todd M., et al. "ORAL02. 01: safety and efficacy of single-agent rovalpituzumab tesirine, a DLL3-targeted ADC, in recurrent or refractory SCLC: topic: medical oncology." Journal of Thoracic Oncology 11.11 (2016): S252-S253.
11. Mizuno Y, Maemura K, Tanaka Y, Hirata A, Futaki S, Hamamoto H, et al. Expression of delta-like 3 is downregulated by aberrant DNA methylation and histone modification in hepatocellular carcinoma. Oncol Rep. 2018;39(5):2209-16.
12. Hamamoto H, Maemura K, Matsuo K, Taniguchi K, Tanaka Y, Futaki S, et al. Delta-like 3 is silenced by HBx via histone acetylation in HBV-associated HCCs. Sci Rep. 2018;8(1):4842.
13 Isobe Y, Sato K, Nishinaga Y, Takahashi K, Taki S, Yasui H, et al. Near infrared photoimmunotherapy targeting DLL3 for small cell lung cancer. EBioMedicine. 2020;52:102632.
14. Giffin MJ, Cooke K, Lobenhofer EK, Estrada J, Zhan J, Deegen P, et al. AMG 757, a Half-Life Extended, DLL3-Targeted Bispecific T-Cell Engager, Shows High Potency and Sensitivity in Preclinical Models of Small-Cell Lung Cancer. Clin Cancer Res. 2021;27(5):1526-37.
15. Owen DH, Giffin MJ, Bailis JM, Smit MD, Carbone DP, He K. DLL3: an emerging target in small cell lung cancer. J Hematol Oncol. 2019;12(1):61.
This web search service is supported by Google Inc.
