 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
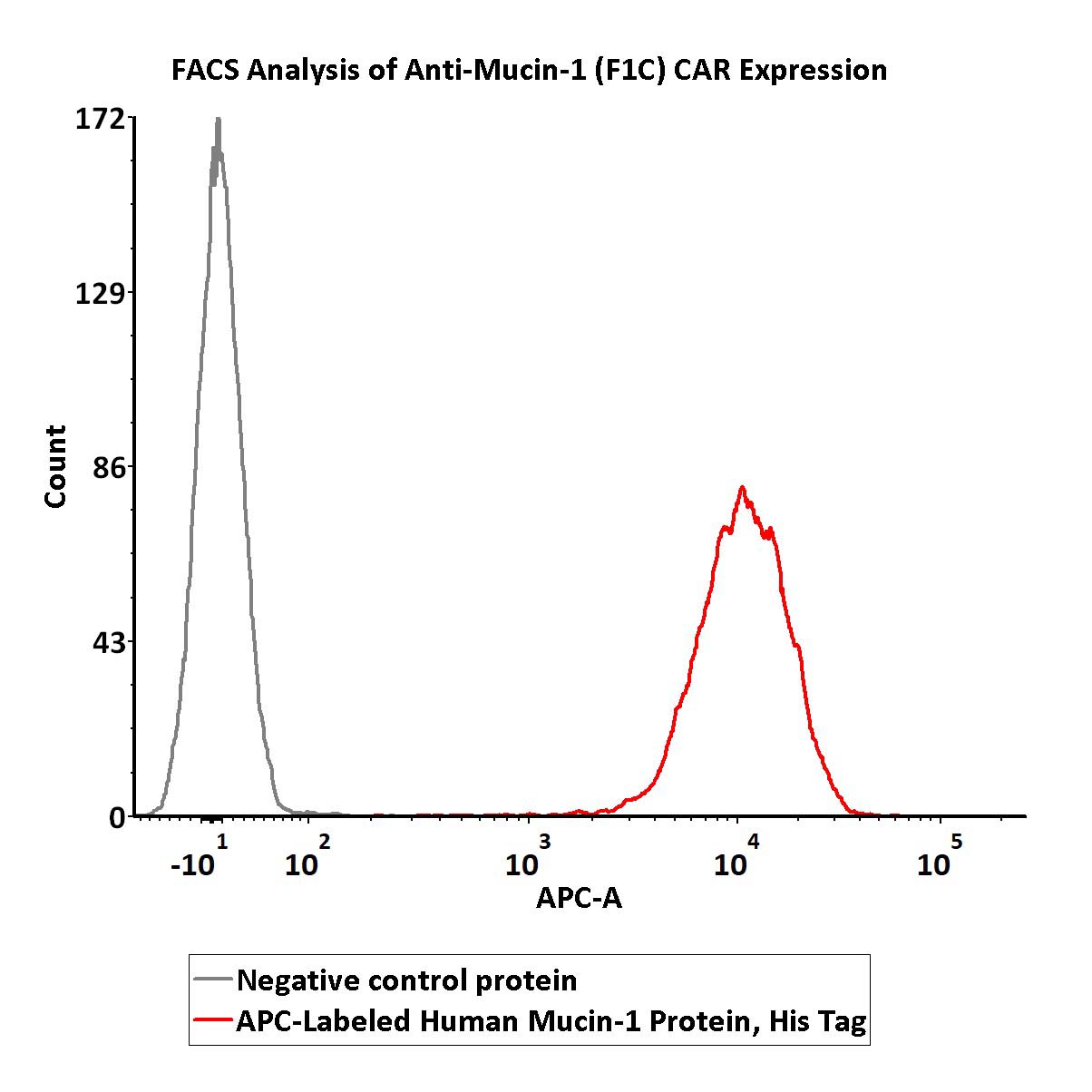
5e5 of anti-Mucin-1 (F1C) CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of APC-Labeled Human Mucin-1 Protein, His Tag (Cat. No. MU1-HA2H8) and negative control protein respectively. APC signal was used to evaluate the binding activity (QC tested).
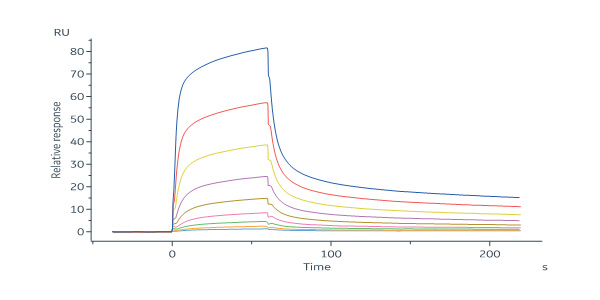
Mouse Mucin-1 (21-535) Protein, His Tag (Cat. No. MU1-M52H4) immobilized on CM5 Chip can bind anti-mMUC1-mAb with an affinity constant of 302 nM as determined in a SPR assay (Biacore 8K) (QC tested).
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Cancer vaccine (BN ImmunoTherapeutics/National Cancer Institute) | CVAC-301; CV-301 | Phase 2 Clinical | Therion Biologics | Ovarian Neoplasms; Cystadenocarcinoma; Intestinal Neoplasms; Solid tumours; Pancreatic Neoplasms; Urinary Bladder Neoplasms; Breast Neoplasms; Prostatic Neoplasms; Colorectal Neoplasms; Carcinoma, Squamous Cell; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Neoplasm Metastasis; Melanoma | Details |
| Anti-MUC1 CAR T cell (Guangzhou Anjie Biomedical) | Phase 2 Clinical | Guangzhou Anjie Biomedical Technology | Esophageal Neoplasms; Breast Neoplasms; Prostatic Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| Anti-MUC1 monoclonal antibody (OncoQuest) | AR20.5; mAb-AR20.5; Anti-MUC1 AR20.5 | Phase 2 Clinical | Altarex | Pancreatic Neoplasms | Details |
| Adenoviral MUC1 vaccine (Etubics) | ETBX-061; Ad5-MUC1 | Phase 2 Clinical | Etubics Corp, Immunitybio Inc, Nantkwest Inc | Chordoma; Melanoma; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Carcinoma, Squamous Cell; Lung Neoplasms; Lymphoma, Non-Hodgkin; Colorectal Neoplasms; Prostatic Neoplasms; Breast Neoplasms; Ovarian Neoplasms; Carcinoma, Transitional Cell; Neoplasms; Small Cell Lung Carcinoma; Triple Negative Breast Neoplasms; Colonic Neoplasms; Pancreatic Neoplasms; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Merkel Cell | Details |
| GO-2032c | GO-203-2C | Phase 2 Clinical | Genus Oncology Llc, Dana-Farber Cancer Institute | Solid tumours; Multiple Myeloma; Leukemia, Myeloid, Acute | Details |
| MUC1-Poly-ICLC | MUC1-poly-ICLC | Phase 2 Clinical | University Of Pittsburgh | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| BLP25 liposome vaccine | BLP-25; L-BLP25; ONO-7165; LP-BLP-25; EMD-531444 | Phase 2 Clinical | Cascadian Therapeutics Inc | Rectal Neoplasms; Small Cell Lung Carcinoma; Multiple Myeloma; Breast Neoplasms; Prostatic Neoplasms; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| DS-3939 | DS-3939; DS-3939a | Phase 2 Clinical | Daiichi Sankyo Inc | Solid tumours; Neoplasm Metastasis | Details |
| BromAc | Phase 2 Clinical | Mucpharm Pty Ltd | Coronavirus Disease 2019 (COVID-19); Pneumonia, Ventilator-Associated | Details | |
| BM7PE | Phase 2 Clinical | Oslo University Hospital | Colorectal Neoplasms | Details | |
| Anti-MUC1 CAR T-cell therapy (Guangzhou Anjie Biomedical Technology/University of Technology Sydney) | Phase 2 Clinical | The First Affiliated Hospital Of Guangdong Pharmaceutical University, Guangzhou Anjie Biomedical Technology, University of Sydney | Carcinoma, Non-Small-Cell Lung | Details | |
| huMNC2-CAR44 | huMNC2-CAR44 | Phase 1 Clinical | Minerva Biotechnologies | Breast Neoplasms | Details |
| Anti MUC 1 chimeric antigen receptor T cell therapy (Innovative Cellular) | Phase 1 Clinical | Innovative Cellular Therapeutics Co Ltd | Pancreatic Neoplasms; Breast Neoplasms; Lung Neoplasms | Details | |
| Gatipotuzumab | GT-MAB-2.5-GEX | Phase 1 Clinical | Nemod, Glycotope Gmbh | Solid tumours; Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms; Fallopian Tube Neoplasms | Details |
| P-MUC1C-ALLO1 | P-MUC1C-ALLO1; P-MUC1C-101 | Phase 1 Clinical | Poseida Therapeutics Inc | Solid tumours; Ovarian Neoplasms; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Pancreatic Neoplasms; Nasopharyngeal Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| MUC1-peptide-DC-CTL (Beijing Doing Biomedical) | Phase 1 Clinical | Beijing Doing Biomedical Co Ltd | Stomach Neoplasms | Details | |
| MUC1-gene-DC-CTL (Beijing Doing Biomedical) | Phase 1 Clinical | Beijing Doing Biomedical Co Ltd | Stomach Neoplasms | Details | |
| Recombinant HSP-MUC1 fusion protein (Newsummit) | Phase 1 Clinical | Shanghai Xinshengyuan Biological Medicine Co Ltd | Breast Neoplasms | Details | |
| DXC-005 | DXC-005 | Phase 1 Clinical | Hangzhou Dac Biotech Company Ltd | Solid tumours | Details |
| M-1231 | M-1231 | Phase 1 Clinical | Emd Serono Research & Development Institute Inc, Merck Serono | Solid tumours; Esophageal Neoplasms; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung | Details |
| ICTCAR-046 | ICTCAR-046 | Clinical | Innovative Cellular Therapeutics Co Ltd | Pancreatic Neoplasms | Details |
| Cancer vaccine (BN ImmunoTherapeutics/National Cancer Institute) | CVAC-301; CV-301 | Phase 2 Clinical | Therion Biologics | Ovarian Neoplasms; Cystadenocarcinoma; Intestinal Neoplasms; Solid tumours; Pancreatic Neoplasms; Urinary Bladder Neoplasms; Breast Neoplasms; Prostatic Neoplasms; Colorectal Neoplasms; Carcinoma, Squamous Cell; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Neoplasm Metastasis; Melanoma | Details |
| Anti-MUC1 CAR T cell (Guangzhou Anjie Biomedical) | Phase 2 Clinical | Guangzhou Anjie Biomedical Technology | Esophageal Neoplasms; Breast Neoplasms; Prostatic Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| Anti-MUC1 monoclonal antibody (OncoQuest) | AR20.5; mAb-AR20.5; Anti-MUC1 AR20.5 | Phase 2 Clinical | Altarex | Pancreatic Neoplasms | Details |
| Adenoviral MUC1 vaccine (Etubics) | ETBX-061; Ad5-MUC1 | Phase 2 Clinical | Etubics Corp, Immunitybio Inc, Nantkwest Inc | Chordoma; Melanoma; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Carcinoma, Squamous Cell; Lung Neoplasms; Lymphoma, Non-Hodgkin; Colorectal Neoplasms; Prostatic Neoplasms; Breast Neoplasms; Ovarian Neoplasms; Carcinoma, Transitional Cell; Neoplasms; Small Cell Lung Carcinoma; Triple Negative Breast Neoplasms; Colonic Neoplasms; Pancreatic Neoplasms; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Merkel Cell | Details |
| GO-2032c | GO-203-2C | Phase 2 Clinical | Genus Oncology Llc, Dana-Farber Cancer Institute | Solid tumours; Multiple Myeloma; Leukemia, Myeloid, Acute | Details |
| MUC1-Poly-ICLC | MUC1-poly-ICLC | Phase 2 Clinical | University Of Pittsburgh | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| BLP25 liposome vaccine | BLP-25; L-BLP25; ONO-7165; LP-BLP-25; EMD-531444 | Phase 2 Clinical | Cascadian Therapeutics Inc | Rectal Neoplasms; Small Cell Lung Carcinoma; Multiple Myeloma; Breast Neoplasms; Prostatic Neoplasms; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| DS-3939 | DS-3939; DS-3939a | Phase 2 Clinical | Daiichi Sankyo Inc | Solid tumours; Neoplasm Metastasis | Details |
| BromAc | Phase 2 Clinical | Mucpharm Pty Ltd | Coronavirus Disease 2019 (COVID-19); Pneumonia, Ventilator-Associated | Details | |
| BM7PE | Phase 2 Clinical | Oslo University Hospital | Colorectal Neoplasms | Details | |
| Anti-MUC1 CAR T-cell therapy (Guangzhou Anjie Biomedical Technology/University of Technology Sydney) | Phase 2 Clinical | The First Affiliated Hospital Of Guangdong Pharmaceutical University, Guangzhou Anjie Biomedical Technology, University of Sydney | Carcinoma, Non-Small-Cell Lung | Details | |
| huMNC2-CAR44 | huMNC2-CAR44 | Phase 1 Clinical | Minerva Biotechnologies | Breast Neoplasms | Details |
| Anti MUC 1 chimeric antigen receptor T cell therapy (Innovative Cellular) | Phase 1 Clinical | Innovative Cellular Therapeutics Co Ltd | Pancreatic Neoplasms; Breast Neoplasms; Lung Neoplasms | Details | |
| Gatipotuzumab | GT-MAB-2.5-GEX | Phase 1 Clinical | Nemod, Glycotope Gmbh | Solid tumours; Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms; Fallopian Tube Neoplasms | Details |
| P-MUC1C-ALLO1 | P-MUC1C-ALLO1; P-MUC1C-101 | Phase 1 Clinical | Poseida Therapeutics Inc | Solid tumours; Ovarian Neoplasms; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Pancreatic Neoplasms; Nasopharyngeal Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| MUC1-peptide-DC-CTL (Beijing Doing Biomedical) | Phase 1 Clinical | Beijing Doing Biomedical Co Ltd | Stomach Neoplasms | Details | |
| MUC1-gene-DC-CTL (Beijing Doing Biomedical) | Phase 1 Clinical | Beijing Doing Biomedical Co Ltd | Stomach Neoplasms | Details | |
| Recombinant HSP-MUC1 fusion protein (Newsummit) | Phase 1 Clinical | Shanghai Xinshengyuan Biological Medicine Co Ltd | Breast Neoplasms | Details | |
| DXC-005 | DXC-005 | Phase 1 Clinical | Hangzhou Dac Biotech Company Ltd | Solid tumours | Details |
| M-1231 | M-1231 | Phase 1 Clinical | Emd Serono Research & Development Institute Inc, Merck Serono | Solid tumours; Esophageal Neoplasms; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung | Details |
| ICTCAR-046 | ICTCAR-046 | Clinical | Innovative Cellular Therapeutics Co Ltd | Pancreatic Neoplasms | Details |
This web search service is supported by Google Inc.
