 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Project Name | Project Stage | Molecule Type | Host Species | Therapeutic Area | Indications |
| BAFFR&CD3 BsAb - 01 | PCC | Hematological Malignancy,AutoImmunity | Blood tumor,Autoimmune disease |
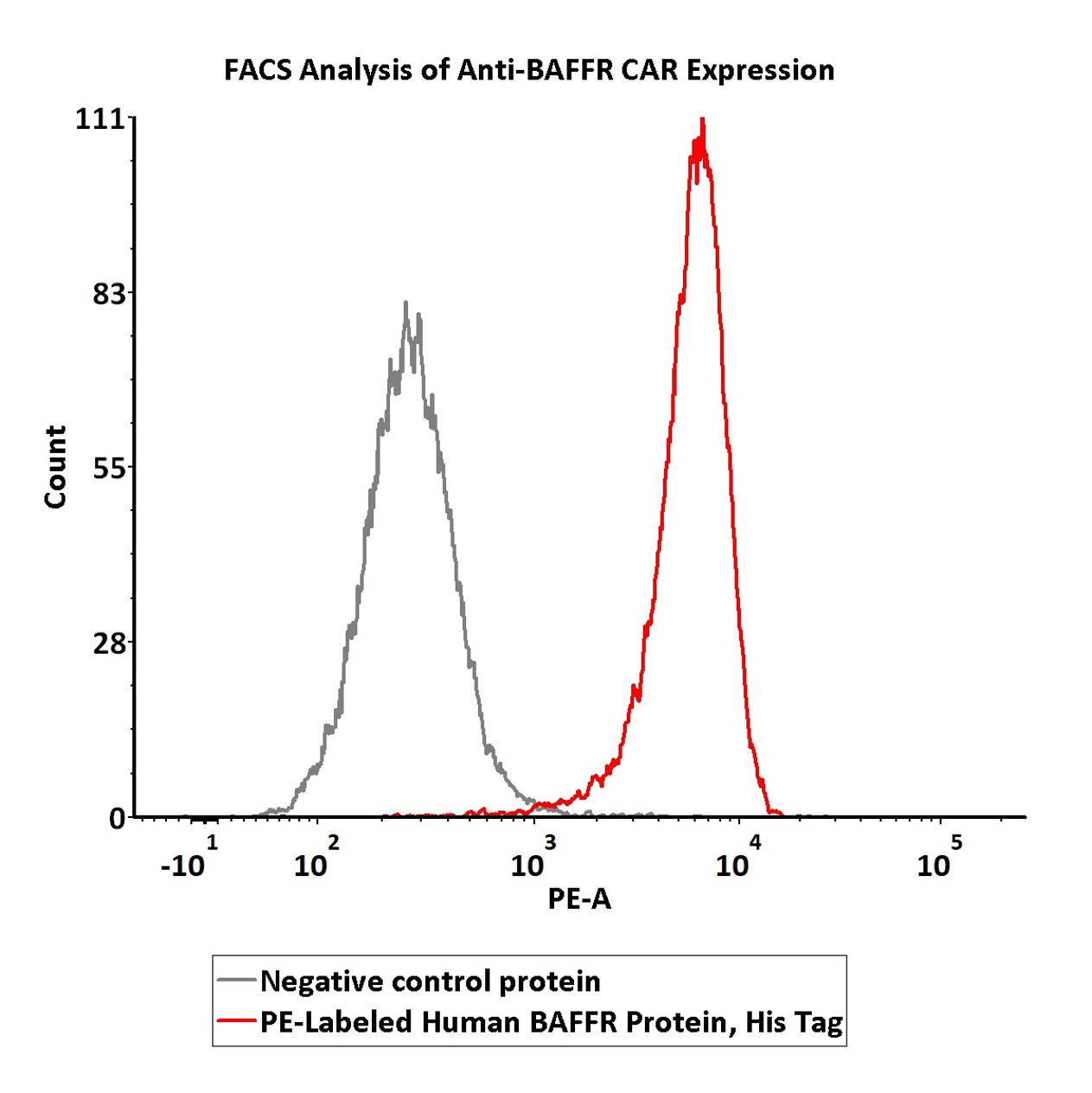
5e5 of anti-BAFFR CAR-293 cells were stained with 100 μL of 1:25 dilution (4 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human BAFFR Protein, His Tag (Cat. No. BAR-HP2H6) and negative control protein respectively. PE signal was used to evaluate the binding activity (QC tested).
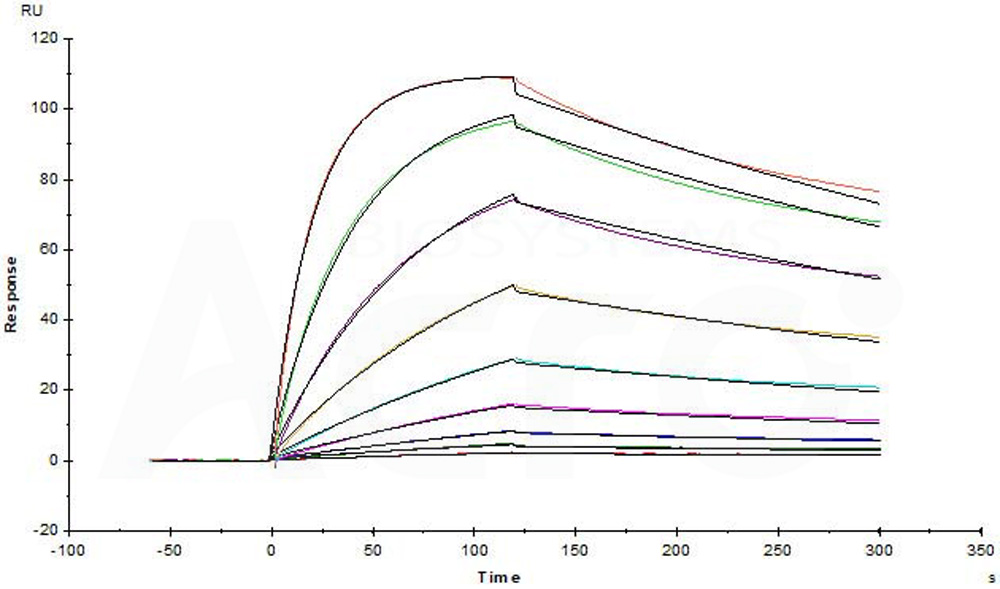
Immobilized Human BAFF Protein, Fc Tag (Cat. No. BAF-H5261) captured on CM5 chip via Anti-human IgG Fc antibodies surface can bind Human BAFFR Protein, Llama IgG2b Fc Tag (Cat. No. BAR-H5258) with an affinity constant of 44.5 nM as determined in a SPR assay (Biacore T200) (Routinely tested).
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Ianalumab | NOV-5; VAY-736 | Phase 3 Clinical | Morphosys Ag, Novartis Pharma Ag | Anemia, Hemolytic, Autoimmune; Pemphigus; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, Non-Hodgkin; Thrombocytopenia; Hepatitis, Autoimmune; Primary mediastinal B cell lymphoma; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Purpura, Thrombocytopenic, Idiopathic; Lupus Erythematosus, Systemic; Multiple Sclerosis; Lupus Nephritis; Idiopathic Pulmonary Fibrosis; Arthritis, Rheumatoid; Sjogren's Syndrome; Hematologic Neoplasms; Lymphoma, B-Cell, Marginal Zone | Details |
| CD19-BAFF Targeted CAR T-cells Therapy(YaKe Biotechnology) | Phase 1 Clinical | Shanghai YaKe Biotechnology Co Ltd, Zhejiang University | Lymphoma, B-Cell; Autoimmune Diseases | Details | |
| ESG-206 | ESG-206 | Phase 1 Clinical | Shanghai Escugen Biotechnology Co Ltd | Lymphoma, B-Cell; Neoplasms; Lymphoma | Details |
| AUR-200 | AUR-200; AUR200 | Phase 1 Clinical | Thunderbolt Pharma Inc | Autoimmune Diseases | Details |
| BAFFR-CAR T cell therapy (PeproMene Bio Inc) | PMB-CT01; PMB-101 | Phase 1 Clinical | PeproMene Bio Inc, City Of Hope National Medical Center | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Biphenotypic, Acute | Details |
| Ianalumab | NOV-5; VAY-736 | Phase 3 Clinical | Morphosys Ag, Novartis Pharma Ag | Anemia, Hemolytic, Autoimmune; Pemphigus; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, Non-Hodgkin; Thrombocytopenia; Hepatitis, Autoimmune; Primary mediastinal B cell lymphoma; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Purpura, Thrombocytopenic, Idiopathic; Lupus Erythematosus, Systemic; Multiple Sclerosis; Lupus Nephritis; Idiopathic Pulmonary Fibrosis; Arthritis, Rheumatoid; Sjogren's Syndrome; Hematologic Neoplasms; Lymphoma, B-Cell, Marginal Zone | Details |
| CD19-BAFF Targeted CAR T-cells Therapy(YaKe Biotechnology) | Phase 1 Clinical | Shanghai YaKe Biotechnology Co Ltd, Zhejiang University | Lymphoma, B-Cell; Autoimmune Diseases | Details | |
| ESG-206 | ESG-206 | Phase 1 Clinical | Shanghai Escugen Biotechnology Co Ltd | Lymphoma, B-Cell; Neoplasms; Lymphoma | Details |
| AUR-200 | AUR-200; AUR200 | Phase 1 Clinical | Thunderbolt Pharma Inc | Autoimmune Diseases | Details |
| BAFFR-CAR T cell therapy (PeproMene Bio Inc) | PMB-CT01; PMB-101 | Phase 1 Clinical | PeproMene Bio Inc, City Of Hope National Medical Center | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Biphenotypic, Acute | Details |
This web search service is supported by Google Inc.
