 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
| CEA-B048 | Human | ClinMax™ Human Soluble NT5E/CD73 ELISA Kit, PRO | |||
| EP-166 | Human | CD73 Inhibitor Screening Kit | |||
| CD3-R52H5 | Rat | Rat CD73 Protein, His Tag (active enzyme) |  |
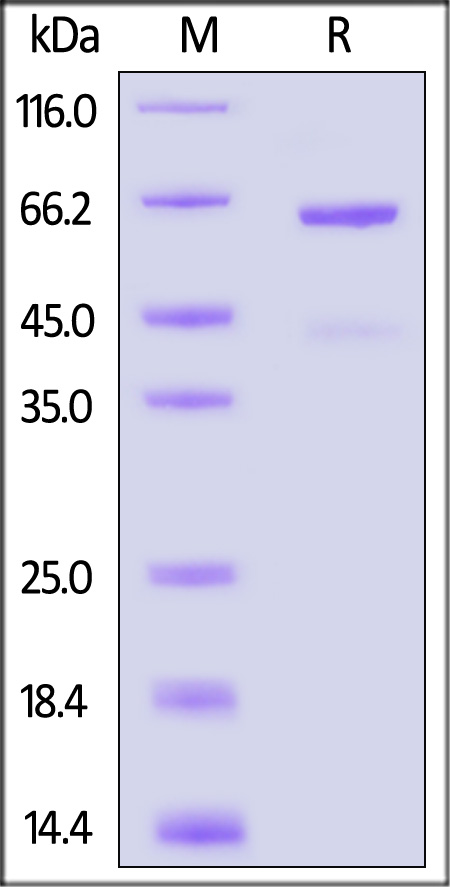
|
|
| CD3-R52H3 | Rabbit | Rabbit CD73 / NT5E Protein, His Tag (active enzyme) |  |
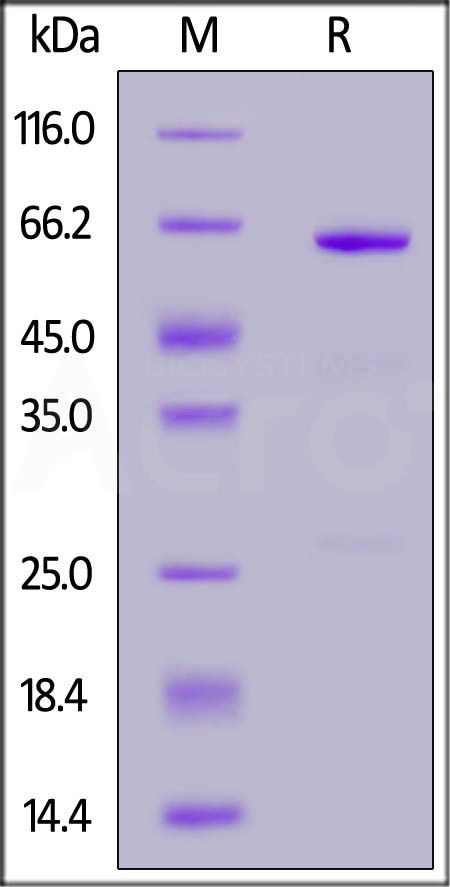
|
|
| CD3-C52H5 | Canine | Canine CD73 / NT5E Protein, His Tag (MALS verified) (active enzyme) |  |
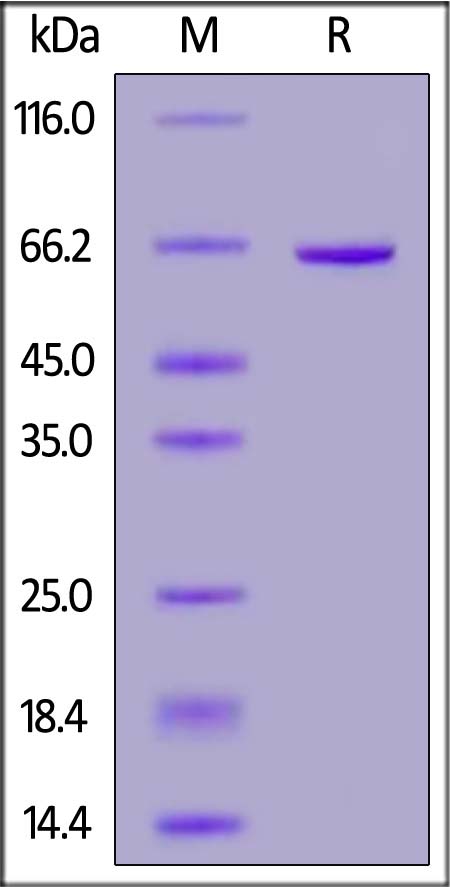
|
|
| CD3-S52H3 | Sus scrofa (Pig) | Sus scrofa CD73 / NT5E Protein, His Tag (active enzyme, MALS verified) |  |
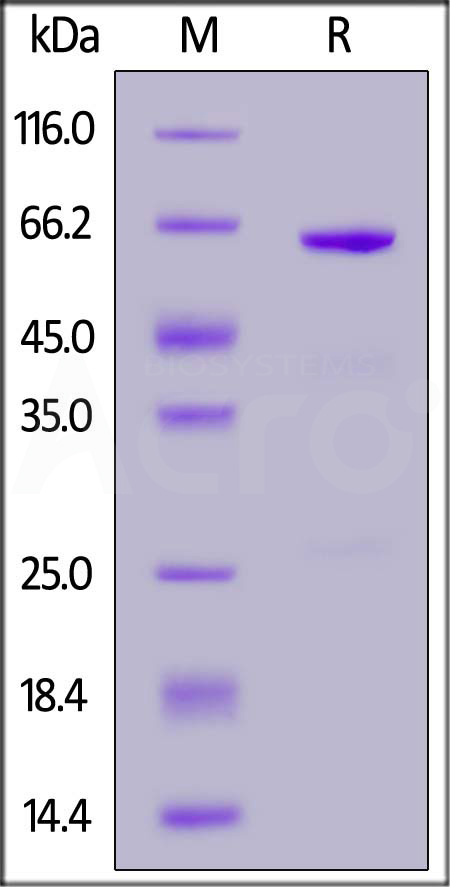
|
|
| MBS-K022 | Human | Human CD73-coupled Magnetic Beads | |||
| CD3-C52H9 | Cynomolgus | Cynomolgus CD73 Protein, His Tag (active enzyme) |  |

|
|
| CD3-H5252 | Human | Human CD73 Protein, Mouse IgG2a Fc Tag (active enzyme) |  |

|
|
| CD3-H82E3 | Human | Biotinylated Human CD73 / NT5E Protein, His,Avitag™ |  |
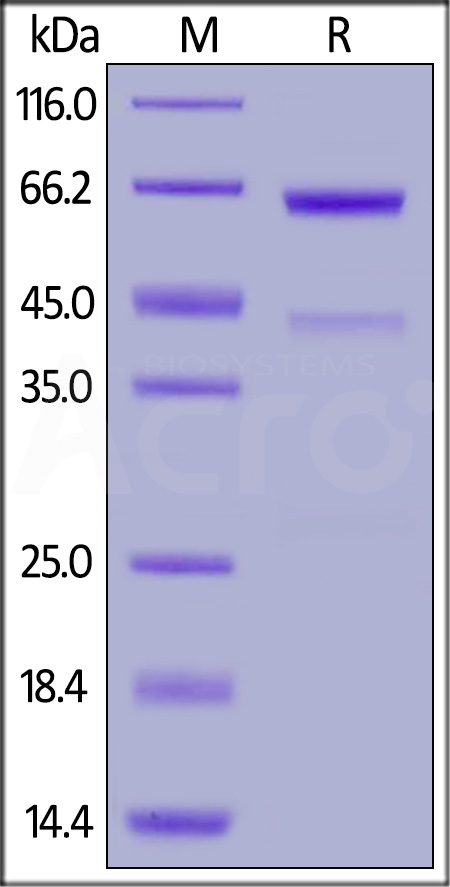
|
|
| CD3-M52H9 | Mouse | Mouse CD73 / NT5E Protein, His Tag (active enzyme) |  |
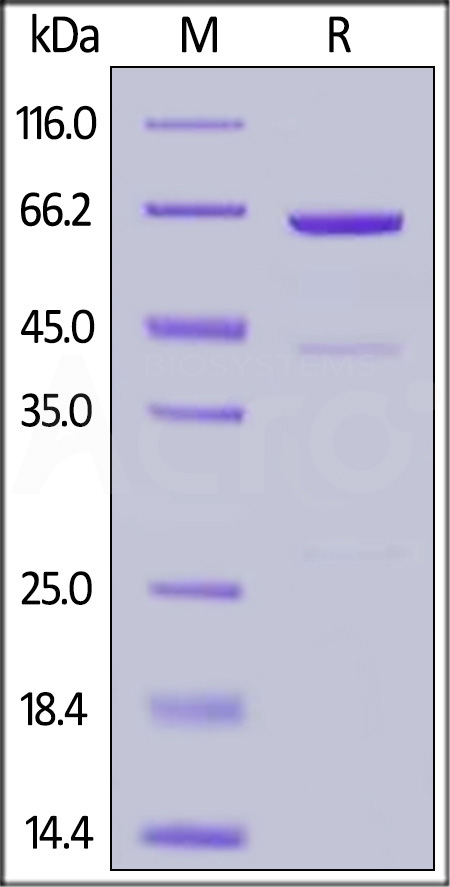
|
|
| CD3-H52H7 | Human | Human CD73 / NT5E Protein, His Tag (HPLC-verified) (active enzyme) |  |
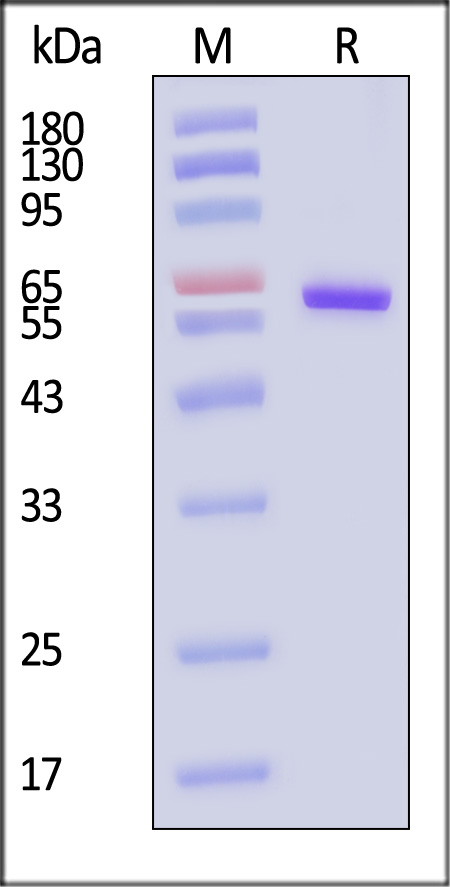
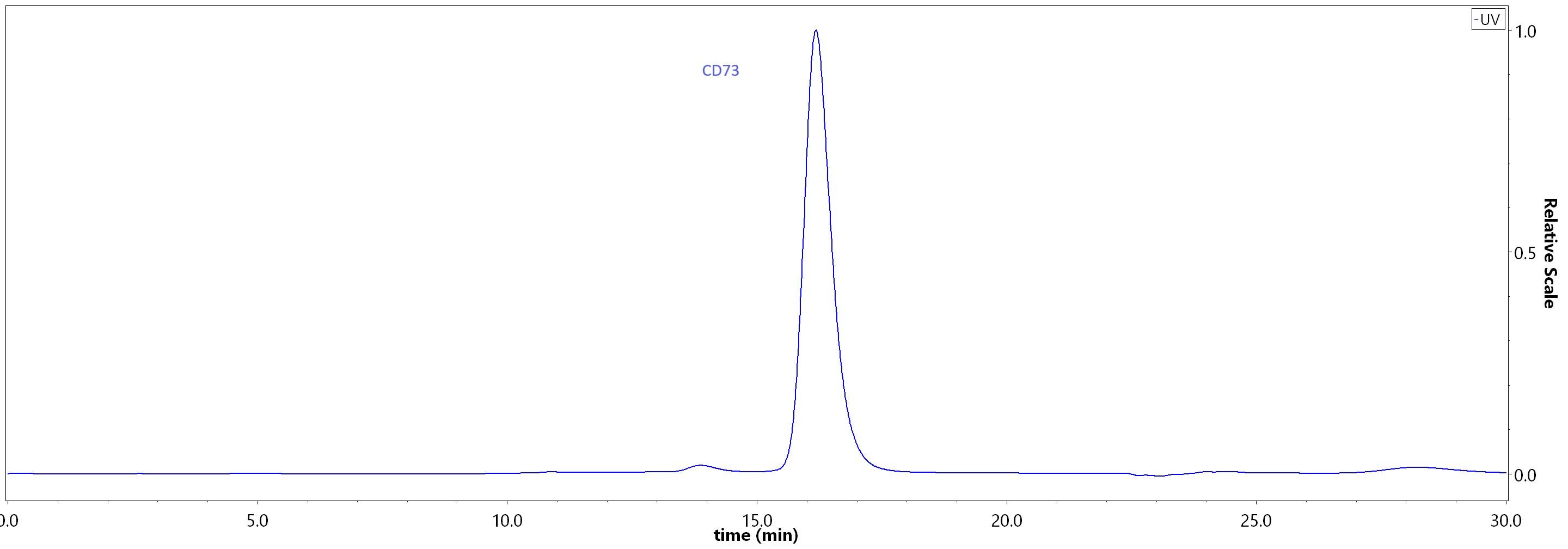
|
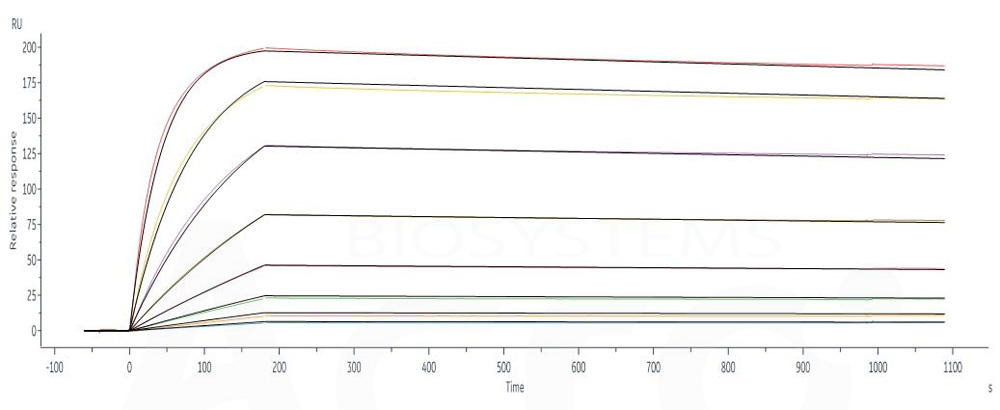
Anti-CD73 antibody (Human IgG1) captured on CM5 chip via Anti-human IgG Fc antibodies surface can bind Human CD73 Protein, His Tag (Cat. No. CD3-H52H7) with an affinity constant of 0.164 nM as determined in a SPR assay (Biacore 8K) (Routinely tested).
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Uliledlimab | TJ-4309; TJ-004309; TJD-5 | Phase 3 Clinical | I-Mab Biopharma Co Ltd | Solid tumours; Ovarian Neoplasms; Head and Neck Neoplasms; Triple Negative Breast Neoplasms; Carcinoma, Ovarian Epithelial; Carcinoma, Non-Small-Cell Lung; Gastrointestinal Neoplasms; Neoplasm Metastasis | Details |
| Oleclumab | MEDI-9447 | Phase 3 Clinical | Medimmune Llc | Solid tumours; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Carcinoma; Hemangiosarcoma; Pancreatic Neoplasms; Triple Negative Breast Neoplasms; Urinary Bladder Neoplasms; Prostatic Neoplasms, Castration-Resistant; Liposarcoma; Prostatic Neoplasms; Osteosarcoma; Colorectal Neoplasms; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma | Details |
| Dalutrafusp alfa | GS-1423; AGEN-1423 | Phase 2 Clinical | Agenus Inc | Liver Neoplasms; Solid tumours; Pancreatic Neoplasms; Colorectal Neoplasms; Carcinoma, Pancreatic Ductal | Details |
| Interferon beta-1a (Faron Pharmaceuticals) | MR-11A8; FP-1201; FP-1201-lyo | Phase 2 Clinical | Faron Pharmaceuticals Ltd | Multiple Organ Failure; Coronavirus Disease 2019 (COVID-19); Respiratory Distress Syndrome, Adult; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Leukemia, B-Cell; Lymphoma, Non-Hodgkin; Acute Lung Injury | Details |
| BMS-986179 | BMS-986179 | Phase 2 Clinical | Bristol-Myers Squibb Company | Solid tumours | Details |
| Mupadolimab | CPX-006; COR-004 (Corvus Pharmaceuticals); CPI-006 | Phase 2 Clinical | Corvus Pharmaceuticals Inc | Papillomavirus Infections; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Lymphoma, Non-Hodgkin; Endometrial Neoplasms; Colorectal Neoplasms; Sarcoma; Prostatic Neoplasms, Castration-Resistant; Urinary Bladder Neoplasms; Solid tumours; Pancreatic Neoplasms; Triple Negative Breast Neoplasms; Coronavirus Disease 2019 (COVID-19); Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Head and Neck Neoplasms; Ovarian Neoplasms | Details |
| Quemliclustat | AB-680 | Phase 2 Clinical | Arcus Biosciences Inc | Biliary Tract Neoplasms; Pancreatic Neoplasms; Prostatic Neoplasms, Castration-Resistant; Cholangiocarcinoma; Prostatic Neoplasms; Bile Duct Neoplasms; Colorectal Neoplasms; Carcinoma, Pancreatic Ductal; Adenocarcinoma; Carcinoma, Non-Small-Cell Lung | Details |
| Dresbuxelimab | AK-119 | Phase 2 Clinical | Akeso Pharmaceuticals Inc | Solid tumours; Coronavirus Disease 2019 (COVID-19); Idiopathic Pulmonary Fibrosis; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| S-095024 | S-095024; S095024 | Phase 2 Clinical | Institut De Recherches Internationales Servier, Servier Bio-Innovation LLC | Carcinoma, Non-Small-Cell Lung | Details |
| HB-0045 | HB-0045; HB0045 | Phase 2 Clinical | Shanghai Huaota Biopharmaceutical Co Ltd | Solid tumours | Details |
| JAB-BX102 | JAB-BX102 | Phase 2 Clinical | Jacobio Pharmaceuticals Co Ltd | Solid tumours | Details |
| LY-3475070 | LY-3475070 | Phase 1 Clinical | Eli Lilly And Company | Neoplasms | Details |
| Anti-CD73 monoclonal antibody (Bristol-Myers Squibb) | Phase 1 Clinical | Bristol-Myers Squibb Company | Neoplasms | Details | |
| Uprevstobart | INCA-00186 | Phase 1 Clinical | Incyte Corp Ltd | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Gastrointestinal Neoplasms | Details |
| HB-0052 | HB-0052; HB0052 | Phase 1 Clinical | Shanghai Huaota Biopharmaceutical Co Ltd | Solid tumours | Details |
| BPI-472372 | BPI-472372 | Phase 1 Clinical | Betta Pharmaceuticals Co Ltd | Solid tumours | Details |
| BB-1709 | BB-1709 | Phase 1 Clinical | Bliss Biopharmaceutical (Hangzhou) Co Ltd | Solid tumours | Details |
| PM-1015 | PM1015; PM-1015 | Phase 1 Clinical | Biotheus Inc | Solid tumours | Details |
| AK-131 | AK-131 | Phase 1 Clinical | Zhongshan Akeso Biopharma Co Ltd | Solid tumours; Neoplasms | Details |
| PT-199 | PT-199 | Phase 1 Clinical | Phanes Therapeutics Inc | Solid tumours; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung | Details |
| IBI-325 | IBI-325 | Phase 1 Clinical | Innovent Biologics(Suzhou) Co Ltd | Solid tumours | Details |
| BR-101 | BR101; BR-101 | Phase 1 Clinical | BioRay Pharmaceutical Co Ltd | Solid tumours | Details |
| CB-708 | CB-708; CB708; ATG-037 | Phase 1 Clinical | Calithera Biosciences Inc | Solid tumours; Neoplasms | Details |
| HLX-23 | HLX-23 | Phase 1 Clinical | Shanghai Henlius Biologics Co Ltd | Solid tumours; Lymphoma | Details |
| ORIC533 | ORIC-533 | Phase 1 Clinical | Oric Pharmaceuticals Inc | Multiple Myeloma; Neoplasm Metastasis | Details |
| IPH-5301 | IPH-5301 | Phase 1 Clinical | Innate Pharma | Ovarian Neoplasms; Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Pancreatic Neoplasms; Neoplasms; Breast Neoplasms; Lung Neoplasms; Endometrial Neoplasms; Neoplasm Metastasis | Details |
| HBM-1007 | HBM1007; R 1007; HBM 1007; HBM-1007 | Phase 1 Clinical | Harbour Biomed | Solid tumours; Neoplasms | Details |
| ABSK-051 | ABSK051 | Phase 1 Clinical | ABbisko Therapeutics Co Ltd | Solid tumours; Neoplasms | Details |
| Uliledlimab | TJ-4309; TJ-004309; TJD-5 | Phase 3 Clinical | I-Mab Biopharma Co Ltd | Solid tumours; Ovarian Neoplasms; Head and Neck Neoplasms; Triple Negative Breast Neoplasms; Carcinoma, Ovarian Epithelial; Carcinoma, Non-Small-Cell Lung; Gastrointestinal Neoplasms; Neoplasm Metastasis | Details |
| Oleclumab | MEDI-9447 | Phase 3 Clinical | Medimmune Llc | Solid tumours; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Carcinoma; Hemangiosarcoma; Pancreatic Neoplasms; Triple Negative Breast Neoplasms; Urinary Bladder Neoplasms; Prostatic Neoplasms, Castration-Resistant; Liposarcoma; Prostatic Neoplasms; Osteosarcoma; Colorectal Neoplasms; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma | Details |
| Dalutrafusp alfa | GS-1423; AGEN-1423 | Phase 2 Clinical | Agenus Inc | Liver Neoplasms; Solid tumours; Pancreatic Neoplasms; Colorectal Neoplasms; Carcinoma, Pancreatic Ductal | Details |
| Interferon beta-1a (Faron Pharmaceuticals) | MR-11A8; FP-1201; FP-1201-lyo | Phase 2 Clinical | Faron Pharmaceuticals Ltd | Multiple Organ Failure; Coronavirus Disease 2019 (COVID-19); Respiratory Distress Syndrome, Adult; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Leukemia, B-Cell; Lymphoma, Non-Hodgkin; Acute Lung Injury | Details |
| BMS-986179 | BMS-986179 | Phase 2 Clinical | Bristol-Myers Squibb Company | Solid tumours | Details |
| Mupadolimab | CPX-006; COR-004 (Corvus Pharmaceuticals); CPI-006 | Phase 2 Clinical | Corvus Pharmaceuticals Inc | Papillomavirus Infections; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Lymphoma, Non-Hodgkin; Endometrial Neoplasms; Colorectal Neoplasms; Sarcoma; Prostatic Neoplasms, Castration-Resistant; Urinary Bladder Neoplasms; Solid tumours; Pancreatic Neoplasms; Triple Negative Breast Neoplasms; Coronavirus Disease 2019 (COVID-19); Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Head and Neck Neoplasms; Ovarian Neoplasms | Details |
| Quemliclustat | AB-680 | Phase 2 Clinical | Arcus Biosciences Inc | Biliary Tract Neoplasms; Pancreatic Neoplasms; Prostatic Neoplasms, Castration-Resistant; Cholangiocarcinoma; Prostatic Neoplasms; Bile Duct Neoplasms; Colorectal Neoplasms; Carcinoma, Pancreatic Ductal; Adenocarcinoma; Carcinoma, Non-Small-Cell Lung | Details |
| Dresbuxelimab | AK-119 | Phase 2 Clinical | Akeso Pharmaceuticals Inc | Solid tumours; Coronavirus Disease 2019 (COVID-19); Idiopathic Pulmonary Fibrosis; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| S-095024 | S-095024; S095024 | Phase 2 Clinical | Institut De Recherches Internationales Servier, Servier Bio-Innovation LLC | Carcinoma, Non-Small-Cell Lung | Details |
| HB-0045 | HB-0045; HB0045 | Phase 2 Clinical | Shanghai Huaota Biopharmaceutical Co Ltd | Solid tumours | Details |
| JAB-BX102 | JAB-BX102 | Phase 2 Clinical | Jacobio Pharmaceuticals Co Ltd | Solid tumours | Details |
| LY-3475070 | LY-3475070 | Phase 1 Clinical | Eli Lilly And Company | Neoplasms | Details |
| Anti-CD73 monoclonal antibody (Bristol-Myers Squibb) | Phase 1 Clinical | Bristol-Myers Squibb Company | Neoplasms | Details | |
| Uprevstobart | INCA-00186 | Phase 1 Clinical | Incyte Corp Ltd | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Gastrointestinal Neoplasms | Details |
| HB-0052 | HB-0052; HB0052 | Phase 1 Clinical | Shanghai Huaota Biopharmaceutical Co Ltd | Solid tumours | Details |
| BPI-472372 | BPI-472372 | Phase 1 Clinical | Betta Pharmaceuticals Co Ltd | Solid tumours | Details |
| BB-1709 | BB-1709 | Phase 1 Clinical | Bliss Biopharmaceutical (Hangzhou) Co Ltd | Solid tumours | Details |
| PM-1015 | PM1015; PM-1015 | Phase 1 Clinical | Biotheus Inc | Solid tumours | Details |
| AK-131 | AK-131 | Phase 1 Clinical | Zhongshan Akeso Biopharma Co Ltd | Solid tumours; Neoplasms | Details |
| PT-199 | PT-199 | Phase 1 Clinical | Phanes Therapeutics Inc | Solid tumours; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung | Details |
| IBI-325 | IBI-325 | Phase 1 Clinical | Innovent Biologics(Suzhou) Co Ltd | Solid tumours | Details |
| BR-101 | BR101; BR-101 | Phase 1 Clinical | BioRay Pharmaceutical Co Ltd | Solid tumours | Details |
| CB-708 | CB-708; CB708; ATG-037 | Phase 1 Clinical | Calithera Biosciences Inc | Solid tumours; Neoplasms | Details |
| HLX-23 | HLX-23 | Phase 1 Clinical | Shanghai Henlius Biologics Co Ltd | Solid tumours; Lymphoma | Details |
| ORIC533 | ORIC-533 | Phase 1 Clinical | Oric Pharmaceuticals Inc | Multiple Myeloma; Neoplasm Metastasis | Details |
| IPH-5301 | IPH-5301 | Phase 1 Clinical | Innate Pharma | Ovarian Neoplasms; Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Pancreatic Neoplasms; Neoplasms; Breast Neoplasms; Lung Neoplasms; Endometrial Neoplasms; Neoplasm Metastasis | Details |
| HBM-1007 | HBM1007; R 1007; HBM 1007; HBM-1007 | Phase 1 Clinical | Harbour Biomed | Solid tumours; Neoplasms | Details |
| ABSK-051 | ABSK051 | Phase 1 Clinical | ABbisko Therapeutics Co Ltd | Solid tumours; Neoplasms | Details |
This web search service is supported by Google Inc.
