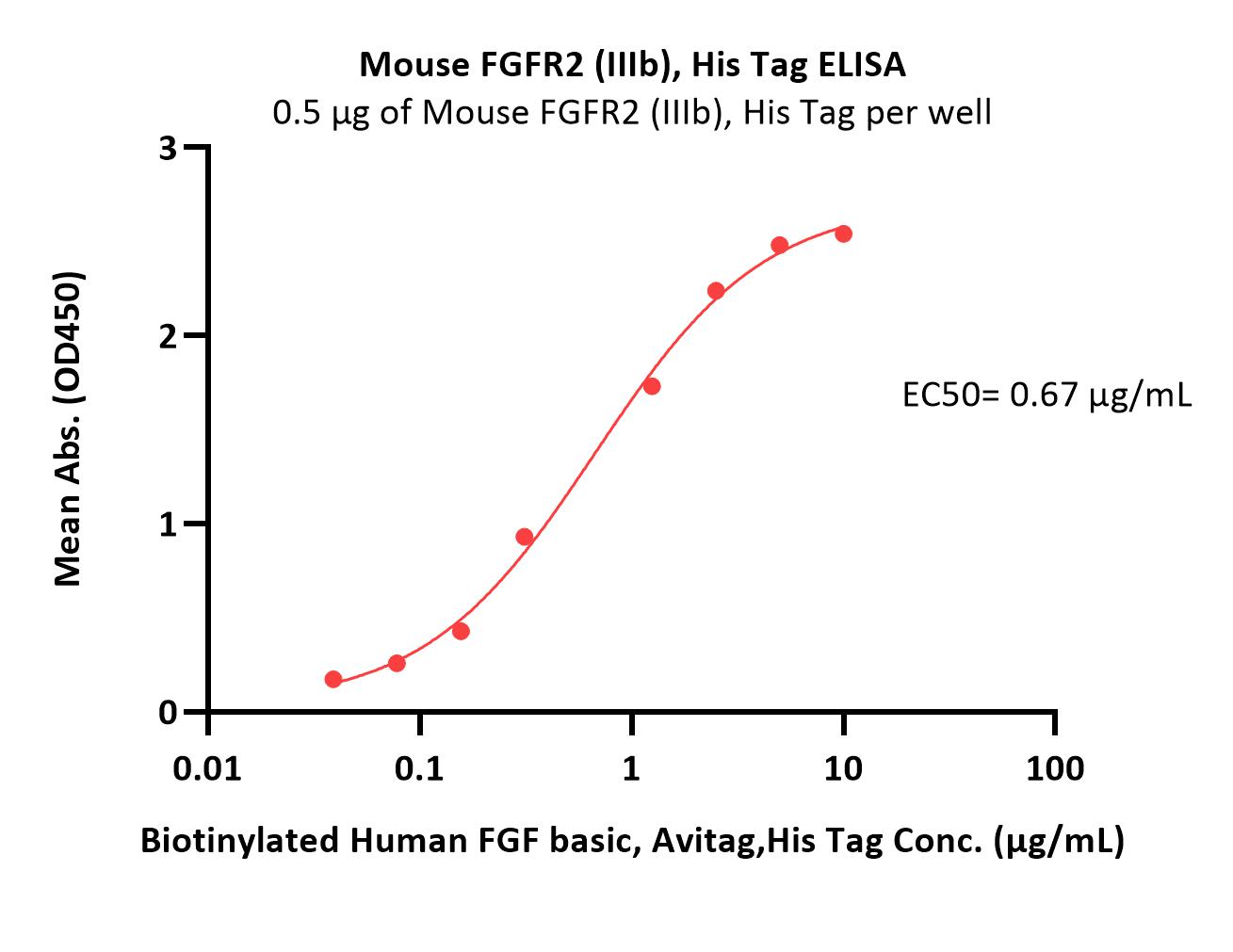
| Lucitanib |
S-80881; AL-3810; CO-3810; E-3810; S-80881-2 |
Phase 3 Clinical |
Advenchen Laboratories Nanjing Ltd |
Solid tumours; Stomach Neoplasms; Small Cell Lung Carcinoma; Nasopharyngeal Carcinoma; Breast Neoplasms; Colorectal Neoplasms; Genital Neoplasms, Female; Thymus Neoplasms; Lung Neoplasms; Carcinoma, Small Cell; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung |
Details
|
| Bemarituzumab |
FPA-144 |
Phase 3 Clinical |
Five Prime Therapeutics Inc |
Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Neoplasms; Carcinoma, Transitional Cell; Urinary Bladder Neoplasms; Digestive System Neoplasms; Carcinoma, Squamous Cell; Adenocarcinoma; Gastrointestinal Neoplasms; Carcinoma, Non-Small-Cell Lung |
Details
|
| Rogaratinib |
BAY-1163877 |
Phase 3 Clinical |
Bayer AG |
Solid tumours; Carcinoma, Transitional Cell; Neoplasms; Urinary Bladder Neoplasms; Sarcoma; Breast Neoplasms; Gastrointestinal Stromal Tumors; Metastatic breast cancer; Carcinoma, Non-Small-Cell Lung |
Details
|
| Fexagratinib |
AZD4547; AZD-4547; ABSK-091; ABSK091 |
Phase 3 Clinical |
Astrazeneca Plc |
Uterine Neoplasms; Multiple Myeloma; Breast Neoplasms; Prostatic Neoplasms; Cholangiocarcinoma; Colorectal Neoplasms; Lung Neoplasms; Carcinoma, Squamous Cell; Glioma; Urinary Bladder Neoplasms; Lymphoma; Endometrial Neoplasms; Thyroid Neoplasms; Melanoma; Carcinoma, Non-Small-Cell Lung; Foodborne Diseases; Adenocarcinoma; Uterine Cervical Neoplasms; Rectal Neoplasms; Solid tumours; Hematologic Neoplasms; Ovarian Neoplasms; Head and Neck Neoplasms; Stomach Neoplasms; Gastrointestinal Diseases; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Liver Neoplasms; Carcinoma, Renal Cell; Carcinoma, Transitional Cell; Neoplasms; Colonic Neoplasms; Carcinoma, Ovarian Epithelial; Pancreatic Neoplasms; Skin Neoplasms |
Details
|
| Infigratinib |
BGJ-398; NVP-BGJ398 |
Phase 3 Clinical |
Novartis Pharma Ag |
Papillomavirus Infections; Adenocarcinoma; Uterine Cervical Neoplasms; Carcinoma, Pancreatic Ductal; Carcinoma, Squamous Cell; Glioma; Oropharyngeal Neoplasms; Gastrointestinal Stromal Tumors; Breast Neoplasms; Cholangiocarcinoma; Urinary Bladder Neoplasms; Central Nervous System Neoplasms; Glioblastoma; Biliary Tract Neoplasms; Neoplasms; Achondroplasia; Nasopharyngeal Neoplasms; Colonic Neoplasms; Pancreatic Neoplasms; Carcinoma, Transitional Cell; Rectal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Esophageal Neoplasms; Hematologic Neoplasms; Solid tumours |
Details
|
| Efruxifermin |
AKR-001; AMG-876; EFX; Fc-FGF21(RGE) |
Phase 3 Clinical |
Amgen Inc |
Metabolic Dysfunction-Associated Steatotic Liver Disease; Diabetes Mellitus |
Details
|
| SC-0011 |
SC-0011 |
Phase 3 Clinical |
Shijiazhuang Zhikang Hongren New Drug Development Co Ltd |
Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Non-Small-Cell Lung |
Details
|
| Zoligratinib |
CH-5183284; FF-284; Debio-1347 |
Phase 2 Clinical |
Chugai Pharmaceutical Co Ltd |
Solid tumours; Breast Neoplasms |
Details
|
| AUR-109 |
ODM-203; AUR-109 |
Phase 2 Clinical |
Orion Corp |
Ovarian Neoplasms; Liver Neoplasms; Solid tumours; Carcinoma, Renal Cell; Urinary Bladder Neoplasms; Pulmonary Fibrosis; Breast Neoplasms; Lung Neoplasms |
Details
|
| Derazantinib |
ARQ-087.2HCl; AQ-14741087; ARQ-087; BAL-087 |
Phase 2 Clinical |
Arqule Inc |
Biliary Tract Neoplasms; Solid tumours; Stomach Neoplasms; Carcinoma, Transitional Cell; Cholangiocarcinoma; Urogenital Neoplasms; Carcinoma, Hepatocellular |
Details
|
| HH185 |
3D-185; 3-D185; HH-185; 3D185 |
Phase 2 Clinical |
ShangHai HaiHe Biopharma Co Ltd, Shanghai Institute Of Materia Medica, Chinese Academy Of Sciences, Shanghai Medicilon Inc |
Solid tumours; Cholangiocarcinoma |
Details
|
| 3HP-2827 |
3HP-2827; 3HP2827 |
Phase 2 Clinical |
3H (Suzhou) Pharmaceuticals Co Ltd |
Solid tumours; Neoplasms |
Details
|
| HMPL-453 |
HMPL-453 |
Phase 2 Clinical |
Hutchison Medipharma Ltd |
Solid tumours; Biliary Tract Neoplasms; Neoplasms, Mesothelial; Mesothelioma; Cholangiocarcinoma |
Details
|
| Lirafugratinib |
RLY-4008 |
Phase 2 Clinical |
Relay Therapeutics Inc |
Cholangiocarcinoma; Translocation, Genetic |
Details
|
| Tasurgratinib |
E-7090 |
Phase 2 Clinical |
Eisai Co Ltd |
Biliary Tract Neoplasms; Solid tumours; Neoplasms; Cholangiocarcinoma; Breast Neoplasms; Hepatic Insufficiency |
Details
|
| Sprifermin |
rhFGF-18; trFGF-18; FGF-18; zFGF-5; Zfgf5; AS-902330 |
Phase 2 Clinical |
Zymogenetics Inc |
Cartilage Diseases; Knee Injuries; Osteoarthritis, Knee |
Details
|
| Gunagratinib |
ICP-192 |
Phase 2 Clinical |
Beijing Tiancheng Pharmaceutical Technology Co Ltd |
Solid tumours; Biliary Tract Neoplasms; Head and Neck Neoplasms; Stomach Neoplasms; Carcinoma, Transitional Cell; Urinary Bladder Neoplasms; Cholangiocarcinoma; Lung Neoplasms |
Details
|
| BPI-17509 |
BPI-17509 |
Phase 1 Clinical |
Betta Pharmaceuticals Co Ltd |
Solid tumours |
Details
|
| TYRA-200 |
TYRA200; TYRA-200 |
Phase 1 Clinical |
Tyra Biosciences Inc |
Biliary Tract Neoplasms; Solid tumours; Cholangiocarcinoma; Bile Duct Neoplasms |
Details
|
| Resigratinib |
KIN-3248; KIN-003 |
Phase 1 Clinical |
Kinnate Biopharma Inc |
Solid tumours; Neoplasms; Carcinoma, Transitional Cell; Cholangiocarcinoma |
Details
|
| Alofanib |
RPT-835 |
Phase 1 Clinical |
Russian Pharmaceutical Technologies Llc |
Stomach Neoplasms |
Details
|
| TT-00434 |
TT-00434 |
Phase 1 Clinical |
TransThera Sciences (Nanjing) Inc |
Solid tumours; Urinary Bladder Neoplasms |
Details
|
| ABSK-121 |
ABSK121-NX; ABSK121 |
Phase 1 Clinical |
ABbisko Therapeutics Co Ltd |
Solid tumours |
Details
|
| ABSK-061 |
ABSK061 |
Phase 1 Clinical |
ABbisko Therapeutics Co Ltd |
Solid tumours |
Details
|
| LY-2874455 |
LY-2874455 |
Phase 1 Clinical |
Eli Lilly And Company |
Neoplasms; Leukemia, Myeloid, Acute |
Details
|
| CPL-304110 |
CPL-304110; CPL-304-110 |
Phase 1 Clinical |
Celon Pharma Sa |
Stomach Neoplasms; Urinary Bladder Neoplasms; Sarcoma; Cholangiocarcinoma; Endometrial Neoplasms; Carcinoma, Squamous Cell; Carcinoma, Non-Small-Cell Lung |
Details
|
| Lucitanib |
S-80881; AL-3810; CO-3810; E-3810; S-80881-2 |
Phase 3 Clinical |
Advenchen Laboratories Nanjing Ltd |
Solid tumours; Stomach Neoplasms; Small Cell Lung Carcinoma; Nasopharyngeal Carcinoma; Breast Neoplasms; Colorectal Neoplasms; Genital Neoplasms, Female; Thymus Neoplasms; Lung Neoplasms; Carcinoma, Small Cell; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung |
Details
|
| Bemarituzumab |
FPA-144 |
Phase 3 Clinical |
Five Prime Therapeutics Inc |
Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Neoplasms; Carcinoma, Transitional Cell; Urinary Bladder Neoplasms; Digestive System Neoplasms; Carcinoma, Squamous Cell; Adenocarcinoma; Gastrointestinal Neoplasms; Carcinoma, Non-Small-Cell Lung |
Details
|
| Rogaratinib |
BAY-1163877 |
Phase 3 Clinical |
Bayer AG |
Solid tumours; Carcinoma, Transitional Cell; Neoplasms; Urinary Bladder Neoplasms; Sarcoma; Breast Neoplasms; Gastrointestinal Stromal Tumors; Metastatic breast cancer; Carcinoma, Non-Small-Cell Lung |
Details
|
| Fexagratinib |
AZD4547; AZD-4547; ABSK-091; ABSK091 |
Phase 3 Clinical |
Astrazeneca Plc |
Uterine Neoplasms; Multiple Myeloma; Breast Neoplasms; Prostatic Neoplasms; Cholangiocarcinoma; Colorectal Neoplasms; Lung Neoplasms; Carcinoma, Squamous Cell; Glioma; Urinary Bladder Neoplasms; Lymphoma; Endometrial Neoplasms; Thyroid Neoplasms; Melanoma; Carcinoma, Non-Small-Cell Lung; Foodborne Diseases; Adenocarcinoma; Uterine Cervical Neoplasms; Rectal Neoplasms; Solid tumours; Hematologic Neoplasms; Ovarian Neoplasms; Head and Neck Neoplasms; Stomach Neoplasms; Gastrointestinal Diseases; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Liver Neoplasms; Carcinoma, Renal Cell; Carcinoma, Transitional Cell; Neoplasms; Colonic Neoplasms; Carcinoma, Ovarian Epithelial; Pancreatic Neoplasms; Skin Neoplasms |
Details
|
| Infigratinib |
BGJ-398; NVP-BGJ398 |
Phase 3 Clinical |
Novartis Pharma Ag |
Papillomavirus Infections; Adenocarcinoma; Uterine Cervical Neoplasms; Carcinoma, Pancreatic Ductal; Carcinoma, Squamous Cell; Glioma; Oropharyngeal Neoplasms; Gastrointestinal Stromal Tumors; Breast Neoplasms; Cholangiocarcinoma; Urinary Bladder Neoplasms; Central Nervous System Neoplasms; Glioblastoma; Biliary Tract Neoplasms; Neoplasms; Achondroplasia; Nasopharyngeal Neoplasms; Colonic Neoplasms; Pancreatic Neoplasms; Carcinoma, Transitional Cell; Rectal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Esophageal Neoplasms; Hematologic Neoplasms; Solid tumours |
Details
|
| Efruxifermin |
AKR-001; AMG-876; EFX; Fc-FGF21(RGE) |
Phase 3 Clinical |
Amgen Inc |
Metabolic Dysfunction-Associated Steatotic Liver Disease; Diabetes Mellitus |
Details
|
| SC-0011 |
SC-0011 |
Phase 3 Clinical |
Shijiazhuang Zhikang Hongren New Drug Development Co Ltd |
Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Non-Small-Cell Lung |
Details
|
| Zoligratinib |
CH-5183284; FF-284; Debio-1347 |
Phase 2 Clinical |
Chugai Pharmaceutical Co Ltd |
Solid tumours; Breast Neoplasms |
Details
|
| AUR-109 |
ODM-203; AUR-109 |
Phase 2 Clinical |
Orion Corp |
Ovarian Neoplasms; Liver Neoplasms; Solid tumours; Carcinoma, Renal Cell; Urinary Bladder Neoplasms; Pulmonary Fibrosis; Breast Neoplasms; Lung Neoplasms |
Details
|
| Derazantinib |
ARQ-087.2HCl; AQ-14741087; ARQ-087; BAL-087 |
Phase 2 Clinical |
Arqule Inc |
Biliary Tract Neoplasms; Solid tumours; Stomach Neoplasms; Carcinoma, Transitional Cell; Cholangiocarcinoma; Urogenital Neoplasms; Carcinoma, Hepatocellular |
Details
|
| HH185 |
3D-185; 3-D185; HH-185; 3D185 |
Phase 2 Clinical |
ShangHai HaiHe Biopharma Co Ltd, Shanghai Institute Of Materia Medica, Chinese Academy Of Sciences, Shanghai Medicilon Inc |
Solid tumours; Cholangiocarcinoma |
Details
|
| 3HP-2827 |
3HP-2827; 3HP2827 |
Phase 2 Clinical |
3H (Suzhou) Pharmaceuticals Co Ltd |
Solid tumours; Neoplasms |
Details
|
| HMPL-453 |
HMPL-453 |
Phase 2 Clinical |
Hutchison Medipharma Ltd |
Solid tumours; Biliary Tract Neoplasms; Neoplasms, Mesothelial; Mesothelioma; Cholangiocarcinoma |
Details
|
| Lirafugratinib |
RLY-4008 |
Phase 2 Clinical |
Relay Therapeutics Inc |
Cholangiocarcinoma; Translocation, Genetic |
Details
|
| Tasurgratinib |
E-7090 |
Phase 2 Clinical |
Eisai Co Ltd |
Biliary Tract Neoplasms; Solid tumours; Neoplasms; Cholangiocarcinoma; Breast Neoplasms; Hepatic Insufficiency |
Details
|
| Sprifermin |
rhFGF-18; trFGF-18; FGF-18; zFGF-5; Zfgf5; AS-902330 |
Phase 2 Clinical |
Zymogenetics Inc |
Cartilage Diseases; Knee Injuries; Osteoarthritis, Knee |
Details
|
| Gunagratinib |
ICP-192 |
Phase 2 Clinical |
Beijing Tiancheng Pharmaceutical Technology Co Ltd |
Solid tumours; Biliary Tract Neoplasms; Head and Neck Neoplasms; Stomach Neoplasms; Carcinoma, Transitional Cell; Urinary Bladder Neoplasms; Cholangiocarcinoma; Lung Neoplasms |
Details
|
| BPI-17509 |
BPI-17509 |
Phase 1 Clinical |
Betta Pharmaceuticals Co Ltd |
Solid tumours |
Details
|
| TYRA-200 |
TYRA200; TYRA-200 |
Phase 1 Clinical |
Tyra Biosciences Inc |
Biliary Tract Neoplasms; Solid tumours; Cholangiocarcinoma; Bile Duct Neoplasms |
Details
|
| Resigratinib |
KIN-3248; KIN-003 |
Phase 1 Clinical |
Kinnate Biopharma Inc |
Solid tumours; Neoplasms; Carcinoma, Transitional Cell; Cholangiocarcinoma |
Details
|
| Alofanib |
RPT-835 |
Phase 1 Clinical |
Russian Pharmaceutical Technologies Llc |
Stomach Neoplasms |
Details
|
| TT-00434 |
TT-00434 |
Phase 1 Clinical |
TransThera Sciences (Nanjing) Inc |
Solid tumours; Urinary Bladder Neoplasms |
Details
|
| ABSK-121 |
ABSK121-NX; ABSK121 |
Phase 1 Clinical |
ABbisko Therapeutics Co Ltd |
Solid tumours |
Details
|
| ABSK-061 |
ABSK061 |
Phase 1 Clinical |
ABbisko Therapeutics Co Ltd |
Solid tumours |
Details
|
| LY-2874455 |
LY-2874455 |
Phase 1 Clinical |
Eli Lilly And Company |
Neoplasms; Leukemia, Myeloid, Acute |
Details
|
| CPL-304110 |
CPL-304110; CPL-304-110 |
Phase 1 Clinical |
Celon Pharma Sa |
Stomach Neoplasms; Urinary Bladder Neoplasms; Sarcoma; Cholangiocarcinoma; Endometrial Neoplasms; Carcinoma, Squamous Cell; Carcinoma, Non-Small-Cell Lung |
Details
|
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now! Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions