
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions

This protein carries a polyhistidine tag at the C-terminus.
The protein has a calculated MW of 107.5 kDa. The protein migrates as 110-130 kDa when calibrated against Star Ribbon Pre-stained Protein Marker under reducing (R) condition (SDS-PAGE) due to glycosylation.
>95% as determined by SDS-PAGE.
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
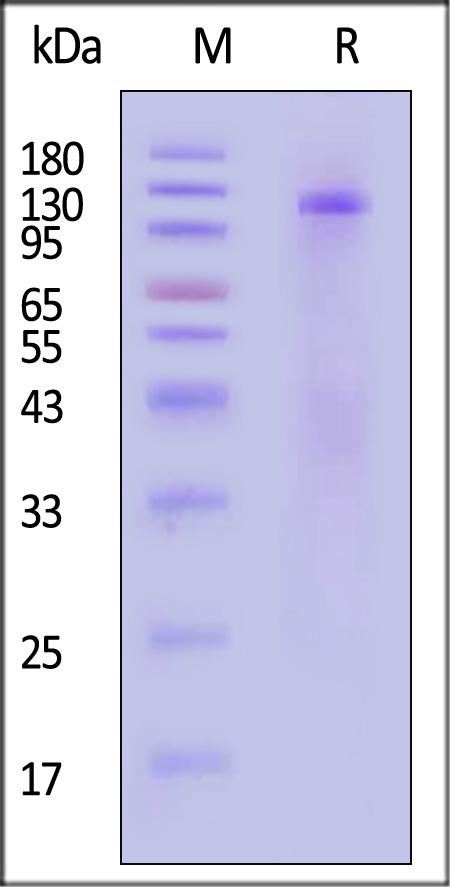
Cat Insulin R (28-956) Protein, His Tag on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95% (With Star Ribbon Pre-stained Protein Marker).
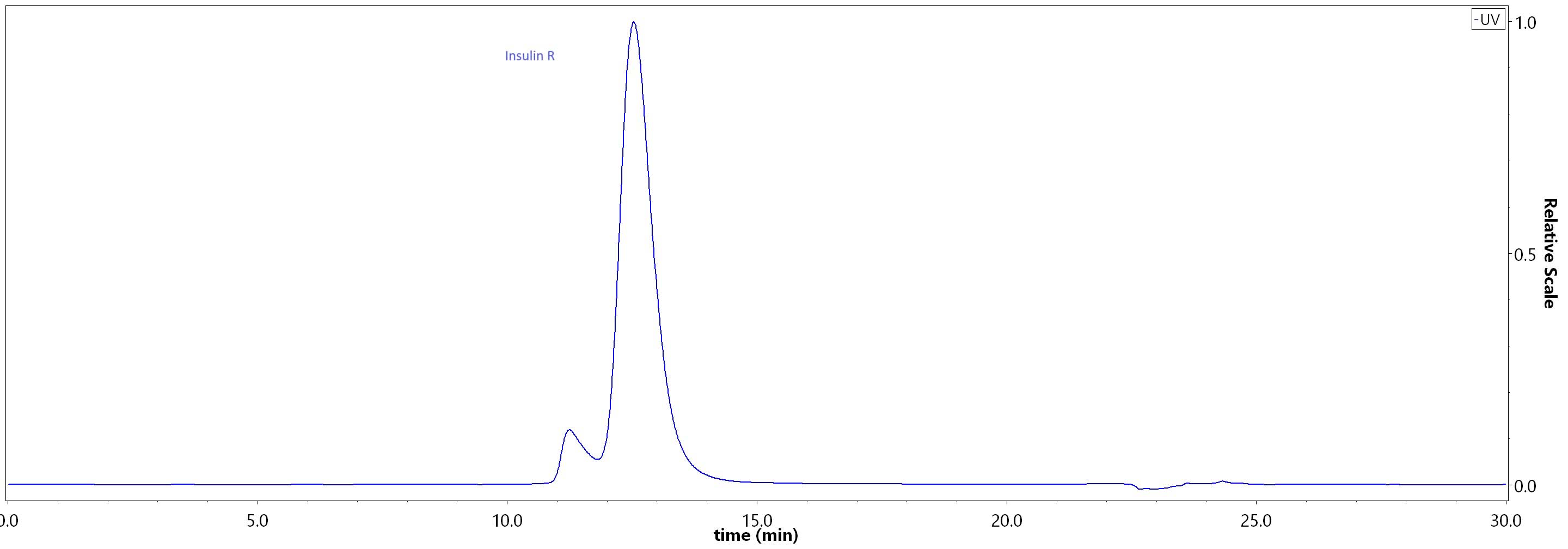
The purity of Cat Insulin R (28-956) Protein, His Tag (Cat. No. INR-C52H3) was greater than 85% as determined by SEC-HPLC.

Explore our catalog of therapeutic antibody solutions to find the right products for you! We are dedicated to delivering solutions designed to help you drive innovation and push the boundaries of what therapeutic antibodies can be.

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

Organoid Toolbox is a collection of organoid solutions including ready-to-use organoids, organoid differentiation kits, and a variety of services to accelerate the progress of your drug development project.

ACROBiosystems developed a series of GMP grade cytokines under the GMP grade quality management system. Those products are all suitable for T/NK cell generation, activation, and proliferation in cell therapy research.

50+ targets designed for CAR detection, including PE/FITC/biotin labeled proteins. The key reagents for CD19 and BCMA were FDA DMF filed which can support your IND, NDA and BLA process.

Full length multi-pass TPs with stabilized structure and high bioactivity for immunization, antibody screening, cell based assay and CAR detection, including hot CD20, Claudin 18.2, CD133, GPRC5D,CCR8, CCR5, etc.

GMP grade cytokines, reagents for cell activation, gene edition, DNA/RNA removal, etc. Particularly focus on product design, quality control and solution-based support to link each phase of your cell and gene therapy journey.

A series of immune checkpoints including classic co-inhibitory and co-stimulatory receptors. The comprehensive catalog contains 100+ targets with various species and tags, and the high-quality proteins are in good batch-to-batch consistency.

To meet the needs of ADCs development, ACROBiosystems can provide: A variety of high-quality target proteins; MMPs/Cathepsin/uPA for cleavable linker; Anti-payload antibodies & anti-idiotypic antibodies for immunogenicity and PK analysis; SPR/BLI analytical and ADA development service.

Comprehensive collection of Fc receptor proteins, including their common variants, which can help expedite your antibody development.

Comprehensive cytokine targets including interleukins, growth factors, chemokines, TNFs, etc. are expressed by HEK293 to ensure their natural structure. Their high purity is verified by SDS-PAGE/HPLC/SEC-MALS and high bioactivity is verified by ELISA/SPR/BLI.

Aneuro provides innovative solutions for neuroscience research. Recombinant proteins, neural factors, pre-formed fibrils, electrophysiological electrodes, as well as Organoid Toolbox all in Aneuro aiming to advance neuroscience research, develop therapeutic interventions, and improve diagnostic methods for neurological diseases.
| English Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Isophane Protamine Recombinant Human Insulin Injection (Shenzhen Kexing Biotech) | Approved | Shenzhen Kexing Biological Engineering Co Ltd | 苏泌啉恩 | Mainland China | Diabetes Mellitus | Shenzhen Kexing Biological Engineering Co Ltd | 2003-02-24 | Diabetes Mellitus | Details | |
| Insulin glargine biosimilar (Tonghua Dongbao Pharmaceutical) | Approved | Tonghua Dongbao Pharmaceutical Co Ltd | 长舒霖 | Mainland China | Diabetes Mellitus | Tonghua Dongbao Pharmaceutical Co Ltd | 2019-12-06 | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Human insulin biosimilar (Square Pharmaceuticals) | Approved | Square Pharmaceuticals Ltd | Ansulin | Bangladesh | Diabetes Mellitus | Square Pharmaceuticals Ltd | 2013-01-01 | Diabetes Mellitus | Details | |
| Recombinant human insulin (United Laboratories International Holdings) | Approved | Zhongshan Branch of Zhuhai United Laboratories Co Ltd | 优思灵, Uslin, 优思灵R | Mainland China | Diabetes Mellitus | Zhongshan Branch of Zhuhai United Laboratories Co Ltd | 2009-11-25 | Diabetes Mellitus | Details | |
| Insulin glargine biosimilar (Getz Pharma) | Approved | Getz Pharma | Basagine | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Details | |||||
| Recombinant human insulin (Tonghua Dongbao Pharmaceutical) | Approved | Tonghua Dongbao Pharmaceutical Co Ltd | 甘舒霖, 甘舒霖R | Mainland China | Diabetes Mellitus | Tonghua Dongbao Pharmaceutical Co Ltd | 1998-01-01 | Diabetes Mellitus | Details | |
| Insulin aspart | NN-1218; INA-X14; Insulin X14 | Approved | Novo Nordisk A/S | Novolog, NovoRapid, NovoLog FlexPen, NovoMix, Fiasp, 诺和锐, NovoRapid/NovoLog | EU | Diabetes Mellitus | Novo Nordisk A/S | 1999-09-07 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Atherosclerosis; Delirium; Hypercholesterolemia; Hypertension; Postoperative Cognitive Complications; Coronary Disease; Cognitive Dysfunction; Alzheimer Disease; Cardiovascular Diseases; Diabetes Mellitus | Details |
| Insulin glargine biosimilar (LG Life Sciences) | Approved | Lg Life Sciences Ltd | Basugine | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Details | |||||
| Insulin biosimilar (Wockhardt) | Approved | Wockhardt Ltd | Wosulin Pen Royale, Wosulin, Wosulin®-R, Wosulin Pen | India | Diabetes Mellitus, Type 1 | Wockhardt Ltd | 2003-10-01 | Diabetes Mellitus, Type 1; Diabetes Mellitus | Details | |
| Insulin detemir | NN-304 | Approved | Novo Nordisk A/S | 诺和平, Levemir | EU | Diabetes Mellitus | Novo Nordisk A/S | 2004-06-01 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Atherosclerosis; Hypercholesterolemia; Hypertension; Diabetes, Gestational; Coronary Disease; Alzheimer Disease; Cognitive Dysfunction; Cardiovascular Diseases; Diabetes Mellitus | Details |
| Insulin biosimilar (Popular Pharmaceuticals) | Approved | Popular Pharmaceuticals | Insul R | Bangladesh | Diabetes Mellitus | Popular Pharmaceuticals | 2013-01-01 | Diabetes Mellitus | Details | |
| Insulin degludec/Liraglutide | NN-9068 | Approved | Novo Nordisk A/S | Xultophy, iDegLira | EU | Diabetes Mellitus, Type 2 | Novo Nordisk A/S | 2014-09-18 | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Imeglimin hydrochloride | EMD-387008; PXL-008 | Approved | Merck KGaA, Darmstadt, Germany | Twymeeg, ツイミーグ | Japan | Diabetes Mellitus, Type 2 | Poxel Sa, Sumitomo Dainippon Pharma Co Ltd | 2021-06-23 | Diabetes Mellitus, Type 2; Heart Diseases; Hepatic Insufficiency | Details |
| Insulin biosimilar (Sedico) | Approved | Sedico | Insulin H bio R | Egypt | Diabetes Mellitus | Sedico | 2013-02-01 | Diabetes Mellitus | Details | |
| Insulin aspart biosimilar (Biocon/Mylan) | MYL-1601D | Approved | Biocon Ltd | EU | Diabetes Mellitus | Biosimilar Collaborations Ireland Ltd | 2021-02-05 | Diabetes Mellitus | Details | |
| Insulin human (rDNA origin, Lilly) | U-500R; S-3300; LY-041001 | Approved | Eli Lilly And Company | Humulin R Kwikpen, Humulin, Humulin R, 优泌淋R, Humulin R Pen | United States | Diabetes Mellitus, Type 2 | Eli Lilly And Company | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Brain Neoplasms; Alzheimer Disease; Cognitive Dysfunction; Intracranial Aneurysm; Diabetes Mellitus | Details | |
| Insulin glargine biosimilar (Wockhardt) | Approved | Wockhardt Bio Ag | Glaritus, Glaritus Cart, Glaritus DispoPen | India | Diabetes Mellitus; Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Wockhardt Ltd | 2013-01-01 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Insulin aspart biosimilar (Tonghua Dongbao Pharmaceutical) | Approved | Tonghua Dongbao Pharmaceutical Co Ltd | Mainland China | Diabetes Mellitus | Tonghua Dongbao Pharmaceutical Co Ltd | 2021-10-19 | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | ||
| Human insulin biosimilar (MJ Biopharm) | Approved | Mj Biopharm Pvt | Biosulin | India | Diabetes Mellitus | Mj Biopharm Pvt | 2005-12-01 | Diabetes Mellitus | Details | |
| Insulin glargine biosimilar(Shandong New Time Pharmaceutical) | Approved | Lunan Pharmaceutical Group Co | Mainland China | Diabetes Mellitus | Shandong New Time Pharmaceutical Co Ltd | 2022-05-10 | Diabetes Mellitus | Details | ||
| Insulin lispro biosimilar (Sanofi) | SAR-342434 | Approved | Sanofi | Admelog | EU | Diabetes Mellitus | Sanofi Winthrop Industrie SA | 2017-07-18 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Insulin biosimilars (Bioton) | Approved | Bioton Sa | Mainland China | Diabetes Mellitus | Bioton Sa | 2013-01-01 | Diabetes Mellitus | Details | ||
| Insulin glargine biosimilar (Biocon) | MYL-1501D | Approved | Fujifilm Corp, Biocon Biopharmaceuticals, Mylan Nv | Semglee, Basalog | India | Diabetes Mellitus | null | 2009-01-01 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Insulin glargine biosimilar (Wanbang Biochemical) | Approved | Wanbang Biopharmaceuticals Co Ltd | Mainland China | Diabetes Mellitus | Wanbang Biopharmaceuticals Co Ltd | 2022-08-30 | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | ||
| Recombinant insulin glargine (Gan & Lee) | GL-GLA | Approved | Gan & Lee Biotech | 长秀霖, Basalin | Mainland China | Diabetes Mellitus | Gan & Lee Pharmaceuticals | 2005-05-10 | Diabetes Mellitus, Type 1; Diabetes Mellitus | Details |
| Insulin degludec/Insulin aspart | NN-1045; NN-5401; NN-1250/Insulin aspart | Approved | Novo Nordisk A/S | Ryzodeg, 诺和佳 | Japan | Diabetes Mellitus; Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Novo Nordisk A/S | 2012-12-25 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Insulin human (rDNA origin, Novo Nordisk) | NN-729 | Approved | Novo Nordisk A/S | Novolin R, 诺和灵R, Mixtard, Actraphane, Insulatard | United States | Diabetes Mellitus, Type 2 | Novo Nordisk Inc | 1991-06-25 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Smoking Cessation; Hyperglycemia; Alcoholism; Parkinson Disease; Alzheimer Disease; Cognitive Dysfunction; Pulmonary Disease, Chronic Obstructive; Lymphoma; Diabetes Mellitus | Details |
| Ceritinib | LDK-378; NVP-LDK378; NVP-LDK378-NX | Approved | Novartis Pharma Ag | 赞可达, Zykadia | EU | Carcinoma, Non-Small-Cell Lung | Novartis Europharm Ltd | 2014-04-29 | Colorectal Neoplasms; Melanoma; Adenocarcinoma; Carcinoma, Non-Small-Cell Lung; Thyroid Neoplasms; Brain metastases; Esophageal adenocarcinoma; Granuloma, Plasma Cell; Lymphoma, Large-Cell, Anaplastic; Hematologic Neoplasms; Hepatic Insufficiency; Cholangiocarcinoma; Pancreatic Neoplasms; Thyroid Carcinoma, Anaplastic; Neoplasms; Glioblastoma; Stomach Neoplasms | Details |
| Isophane Protamine Recombinant Human Insulin biosimilar (Popular Pharmaceuticals) | Approved | Popular Pharmaceuticals | Insulin N | Bangladesh | Diabetes Mellitus | Popular Pharmaceuticals | 2013-01-01 | Diabetes Mellitus | Details | |
| Insulin glargine | U-300; Gla-100; Gla-300; Hoe-901; Hoe-71GT; HOE901-U300 | Approved | Sanofi | 来得时, Lantus, Toujeo, Lantus XR, Optisulin | United States | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Sanofi-Aventis U.S. Llc | 2000-04-20 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Atherosclerosis; Diabetic Ketoacidosis; Hyperglycemia; Hypercholesterolemia; Hypertension; Coronary Disease; Cardiovascular Diseases; Diabetes Mellitus | Details |
| Insulin human (inhalation powder) | SAR-439065 | Approved | Mannkind Corporation | Technosphere Insulin System, Afrezza, Afresa | United States | Diabetes Mellitus, Type 2 | Mannkind Corporation | 2014-06-27 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Hyperglycemia; Asthma; Pulmonary Disease, Chronic Obstructive; Diabetes Mellitus | Details |
| Insulin human | HR-1799 | Approved | Sanofi Deutschland | Insulin Human Winthrop | EU | Diabetes Mellitus; Diabetic Coma; Diabetic Ketoacidosis | Sanofi-Aventis Deutschland Gmbh | 1997-02-21 | Diabetic Coma; Diabetic Ketoacidosis; Glaucoma; Diabetes Mellitus | Details |
| Insulin glargine biosimilar (Incepta Pharmaceuticals) | Approved | Incepta Pharmaceuticals Ltd | Bangladesh | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Incepta Pharmaceuticals Ltd | 2012-08-01 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Details | ||
| Insulin aspart biosimilar (Sanofi) | SAR-341402; SAR-Asp | Approved | Sanofi | Truvelog | EU | Diabetes Mellitus | Sanofi Winthrop Industrie SA | 2020-06-25 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Recombinant lispro insulin biosimilar (Gan & Lee) | Approved | Gan & Lee Pharmaceuticals | Prandilin, 速秀霖 | Mainland China | Diabetes Mellitus | Gan & Lee Pharmaceuticals | 2006-01-19 | Diabetes Mellitus | Details | |
| Rinsulin NPH (Geropharm) | Approved | Geropharm | Rinsulin NPH | Russian Federation | Diabetes Mellitus | Geropharm | 2012-10-01 | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Insulin glargine biosimilar (Lilly/Boehringer Ingelheim) | LY-2963016; LY2963016-U-100; LY2963016-U-200 | Approved | Boehringer Ingelheim Gmbh, Eli Lilly And Company | Basaglar, Abasaglar, Rezvoglar, Abasria | EU | Diabetes Mellitus | Eli Lilly Nederland BV | 2014-09-09 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Hyperglycemia; Renal Insufficiency, Chronic; Diabetes Mellitus | Details |
| Oral insulin (Generex Biotechnology) | Approved | Generex Biotechnology Corp | Oralin, Oralgen, Oral-lyn, Recosulin | Ecuador | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | null | 2005-01-01 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Details | |
| Insul 30/70 biosimilar (Popular Pharmaceuticals) | Approved | Popular Pharmaceuticals | Insul 30-70 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes, Gestational | Details | |||||
| Recombinant Human Insulin (Wanbang Pharma) | Approved | Wanbang Biopharmaceuticals Co Ltd | Mainland China | Diabetes Mellitus | Wanbang Biopharmaceuticals Co Ltd | 2003-08-29 | Diabetes Mellitus | Details | ||
| Insulin degludec | NN-1250; Insulin-454 | Approved | Novo Nordisk A/S | 诺和达, Tresiba | Japan | Diabetes Mellitus, Type 2; Diabetes Mellitus; Diabetes Mellitus, Type 1 | Novo Nordisk A/S | 2012-09-28 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Insulin glulisine | HMR-1964 | Approved | Sanofi | 艾倍得, Apidra | United States | Diabetes Mellitus | Sanofi-Aventis U.S. Llc | 2004-04-16 | Diabetes Mellitus, Type 1; Leukemia; Diabetes Mellitus, Type 2; Heart Failure; Myocardial Infarction; Down Syndrome; Hyperglycemia; Cardiovascular Diseases; Cognitive Dysfunction; Alzheimer Disease; Diabetes Mellitus; Respiratory Insufficiency | Details |
| Insulin biosimilar (Julphar) | Approved | Julphar (Gulf Pharmaceutical Industries) | Julphar Insulin R | Tunisia | Diabetes Mellitus | Julphar (Gulf Pharmaceutical Industries) | 2013-10-01 | Diabetes Mellitus | Details | |
| Insulin lispro protamine(25R) (Lilly ) | Approved | Lilly Suzhou Pharmaceutical Co Ltd | Diabetes Mellitus | Details | ||||||
| Insulin Aspart 50(Tonghua Dongbao Pharmaceutical) | Approved | Tonghua Dongbao Pharmaceutical Co Ltd | Mainland China | Diabetes Mellitus | Tonghua Dongbao Pharmaceutical Co Ltd | 2022-11-08 | Diabetes Mellitus | Details | ||
| Insulin Aspart 30(onghua Dongbao Pharmaceutical) | Approved | Tonghua Dongbao Pharmaceutical Co Ltd | Mainland China | Diabetes Mellitus | Tonghua Dongbao Pharmaceutical Co Ltd | 2022-11-08 | Diabetes Mellitus | Details | ||
| Recombinant insulin aspart biosimilar (Yichang Hec Changjiang Pharmaceutical) | Approved | Yichang Hec Changjiang Pharmaceutical Co Ltd | Mainland China | Diabetes Mellitus | Yichang Hec Changjiang Pharmaceutical Co Ltd | 2022-10-11 | Diabetes Mellitus | Details | ||
| Insulin Aspart 30 | Approved | Novo Nordisk A/S | 诺和锐30 | Mainland China | Diabetes Mellitus | Novo Nordisk A/S | 2009-09-30 | Diabetes Mellitus | Details | |
| Isophane Protamine Human Insulin(30R)(Gan & Lee ) | Approved | Gan & Lee Pharmaceuticals | Mainland China | Diabetes Mellitus | Gan & Lee Pharmaceuticals | 2021-05-19 | Diabetes Mellitus | Details | ||
| Isophane Protamine Human Insulin(50R)(Zhuhai United Laboratories ) | Approved | Zhongshan Branch of Zhuhai United Laboratories Co Ltd | 诺和灵50R | Mainland China | Diabetes Mellitus | Zhongshan Branch of Zhuhai United Laboratories Co Ltd | 2010-12-31 | Diabetes Mellitus | Details | |
| Insulin human (rDNA) biosimilar (Baxter) | Approved | Baxter International Inc | Inpremzia, Myxredlin | United States | Diabetes Mellitus | Baxter Healthcare Corp | 2019-06-20 | Diabetes Mellitus | Details | |
| Insulin aspart biosimilar (Hisun Pharma) | Approved | Zhejiang Hisun Pharmaceutical Co Ltd | Mainland China | Diabetes Mellitus | Zhejiang Hisun Pharmaceutical Co Ltd | 2021-09-13 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | ||
| Isophane Protamine Human Insulin(30R)(Hec Pharm) | Approved | Hec Pharm Co Ltd | 诺和灵30R | Mainland China | Diabetes Mellitus | Hec Pharm Co Ltd | 2023-09-19 | Diabetes Mellitus | Details | |
| Insulin Aspart 30 biosimilar (Jilin Huisheng Biological) | Approved | Jilin Huisheng Biopharmaceutical Co Ltd | Mainland China | Diabetes Mellitus | Jilin Huisheng Biopharmaceutical Co Ltd | 2023-12-26 | Diabetes Mellitus | Details | ||
| Insulin aspart biosimilar(Jilin Huisheng Biological) | Approved | Jilin Huisheng Biopharmaceutical Co Ltd | Mainland China | Diabetes Mellitus | Jilin Huisheng Biopharmaceutical Co Ltd | 2023-12-26 | Diabetes Mellitus | Details | ||
| Isophane Protamine Human Insulin(50R)(Novo Nordisk ) | Approved | Novo Nordisk A/S | 诺和灵50R | Mainland China | Diabetes Mellitus | Novo Nordisk (China)Pharmaceuticals Co Ltd | 2019-11-27 | Diabetes Mellitus | Details | |
| Isophane Protamine Human Insulin(30R)(Novo Nordisk ) | Approved | Novo Nordisk A/S | 诺和灵30R | Mainland China | Diabetes Mellitus | Novo Nordisk A/S | 1999-07-28 | Diabetes Mellitus | Details | |
| Insulin Aspart 50 biosimilar (Jilin Huisheng Biological) | Approved | Jilin Huisheng Biopharmaceutical Co Ltd | Mainland China | Diabetes Mellitus | Jilin Huisheng Biopharmaceutical Co Ltd | 2023-12-26 | Diabetes Mellitus | Details | ||
| Insulin biosimilar (Harvest Moon Pharmaceuticals) | Approved | Harvest Moon | Diabetes Mellitus | Details | ||||||
| 30/70 Mixture Recombinant Human Insulin(Tonghua Dongbao Pharmaceutical) | Approved | Tonghua Dongbao Pharmaceutical Co Ltd | 甘舒霖30R | Mainland China | Diabetes Mellitus | Tonghua Dongbao Pharmaceutical Co Ltd | 2002-05-18 | Diabetes Mellitus | Details | |
| Mixed Protamine Human Insulin(40R)(Tonghua Dongbao Pharmaceutical) | Approved | Tonghua Dongbao Pharmaceutical Co Ltd | 甘舒霖N | Mainland China | Diabetes Mellitus | Tonghua Dongbao Pharmaceutical Co Ltd | 1999-01-01 | Diabetes Mellitus | Details | |
| 50/50 Mixture Recombinant Human Insulin(Tonghua Dongbao Pharmaceutical) | Approved | Tonghua Dongbao Pharmaceutical Co Ltd | 甘舒霖50R | Mainland China | Diabetes Mellitus | Tonghua Dongbao Pharmaceutical Co Ltd | 2008-02-04 | Diabetes Mellitus | Details | |
| Insulin Aspart 50 | Approved | Novo Nordisk A/S | 诺和锐50 | Mainland China | Diabetes Mellitus | Novo Nordisk A/S | 2012-02-10 | Diabetes Mellitus | Details | |
| Insulin aspart biosimilar (Gan & Lee) | GL-ASP | Approved | Gan & Lee Biotech | Rapilin®30, Rapilin30, 锐秀霖30 | Mainland China | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Gan & Lee Biotech | 2020-06-05 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Isophane Protamine Human Insulin(30R)(Lilly) | Approved | Eli Lilly And Company | Mainland China | Diabetes Mellitus | Eli Lilly And Company | Diabetes Mellitus | Details | |||
| Insulin lispro protamine biosimilar(25R) (Gan & Lee) | Approved | Gan & Lee Biotech | Prandilin25, 速秀霖25 | Mainland China | Diabetes Mellitus | Gan & Lee Pharmaceuticals | 2014-05-09 | Diabetes Mellitus | Details | |
| Insulin lispro | LY-900027; LY-275585; Lys-B28; Pro-B29; LYSPRO; LY-900014 | Approved | Eli Lilly And Company | Humalog, 优泌乐, Eglucent, Liprolog, Lyumjev, Liumjev | EU | Diabetes Mellitus | Eli Lilly Nederland BV | 1996-04-30 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Insulin Resistance; Diabetic Neuropathies; Diabetes Mellitus; Hyperlipidemias | Details |
| Recombinant human insulin (Biocon) | Approved | Biocon Ltd | Insugen | India | Diabetes Mellitus | Biocon Ltd | 2004-11-10 | Diabetes Mellitus | Details | |
| Isophane Protamine Human Insulin(40R)(Tonghua Dongbao) | Approved | Tonghua Dongbao Pharmaceutical Co Ltd | Diabetes Mellitus | Details | ||||||
| Insulin glargine biosimilar (Zhuhai United) | Approved | Zhuhai United Laboratories Co Ltd | Mainland China | Diabetes Mellitus | Zhuhai United Laboratories Co Ltd | 2016-12-23 | Diabetes Mellitus | Details | ||
| Isophane Insulin(Polish Society of Diabetology) | Approved | Polish Society of Diabetology | Diabetes Mellitus | Details | ||||||
| Insulin human(Organon France) | Approved | Organon France | EU | Diabetes Mellitus | Organon France | 1999-11-03 | Diabetes Mellitus | Details | ||
| Isophane Protamine Recombinant Human Insulin(Novo Nordisk) | Approved | Novo Nordisk (China)Pharmaceuticals Co Ltd | 诺和灵N | Mainland China | Diabetes Mellitus | Novo Nordisk (China)Pharmaceuticals Co Ltd | 2016-06-16 | Diabetes Mellitus | Details | |
| Isophane Protamine Recombinant Human Insulin (BIOTON) | Approved | Bioton Sa | Mainland China | Diabetes Mellitus | Bioton Sa | 2013-03-29 | Diabetes Mellitus | Details | ||
| Insulin Glargine biosimilar (Liaoning Bo'Ao Bio-Pharmaceutical) | Approved | Liaoning Bo'Ao Bio-Pharmaceutical Co Ltd | Mainland China | Diabetes Mellitus | Liaoning Bo'Ao Bio-Pharmaceutical Co Ltd | 2023-12-05 | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | ||
| Insulin Aspart 30 (Yichang Hec Changjiang Pharmaceutical) | Approved | Yichang Hec Changjiang Pharmaceutical Co Ltd | Mainland China | Diabetes Mellitus | Yichang Hec Changjiang Pharmaceutical Co Ltd | 2022-11-01 | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | ||
| Isophane Protamine Recombinant Human Insulin (Hefei Tianmai Biotechnology) | Approved | Hefei Tianmai Biotechnology Development Co Ltd | Mainland China | Diabetes Mellitus | Hefei Tianmai Biotechnology Development Co Ltd | 2019-09-05 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | ||
| Recombinant human insulin (Hefei Tianmai Biotechnology) | Approved | Hefei Tianmai Biotechnology Development Co Ltd | Mainland China | Diabetes Mellitus | Hefei Tianmai Biotechnology Development Co Ltd | 2018-04-28 | Diabetes Mellitus | Details | ||
| Lixisenatide/Insulin glargine | HOE-901/AVE-0010 | Approved | Sanofi | Soliqua, iGlarLixi, Suliqua | United States | Diabetes Mellitus, Type 2 | Sanofi-Aventis U.S. Llc | 2016-11-21 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Details |
| Insulin icodec | 148-0287-A; LAI-287; Long-acting basal insulin analogue; NN-1436; Insulin-287; NNC-0148-0000-0287; NNC0148-0287 C; OI-287GT; NN-1956 | Approved | Novo Nordisk A/S | AWIQLI, Awiqli | Canada | Diabetes Mellitus | Novo Nordisk Canada Inc | 2024-03-12 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Insulin lispro biosimilar (Wanbang Biopharma) | Approved | Wanbang Biopharmaceuticals Co Ltd | Mainland China | Diabetes Mellitus | Wanbang Biopharmaceuticals Co Ltd | 2022-01-25 | Diabetes Mellitus | Details | ||
| Recombinant human insulin (Shenzhen Kexing Biotech) | Approved | Shenzhen Kexing Biological Engineering Co Ltd | 苏泌啉, Su Bi Lin | Mainland China | Diabetes Mellitus | Shenzhen Kexing Biological Engineering Co Ltd | 2002-06-10 | Diabetes Mellitus | Details | |
| Recombinant insulin (Horizon Pharma/Ibatech) | Approved | Savient Pharmaceuticals Inc | Poland | Diabetes Mellitus, Type 1 | null | 2002-07-01 | Diabetes Mellitus, Type 1 | Details | ||
| Rosinsulin | Approved | Medsintez | Diabetes Mellitus | Details | ||||||
| Isophane Insulin | Approved | Wanbang Biopharmaceuticals Co Ltd | 万苏林 | Mainland China | Diabetes Mellitus | Wanbang Biopharmaceuticals Co Ltd | 1997-01-01 | Diabetes Mellitus | Details | |
| Recombinant human insulin (Incepta) | Approved | Incepta Pharmaceuticals Ltd | Maxsulin | Bangladesh | Diabetes Mellitus | Incepta Pharmaceuticals Ltd | 2012-01-01 | Diabetes Mellitus | Details | |
| Recombinant human insulin (SciGen) | Approved | Generex Biotechnology Corp | Recojet | India | Diabetes Mellitus | null | 2004-12-01 | Diabetes Mellitus | Details | |
| Insulin aspart biosimilar (Zhuhai United Laboratories) | Approved | Zhuhai United Laboratories Co Ltd | 联邦优倍灵, Ublin | Mainland China | Diabetes Mellitus | Zhuhai United Laboratories Co Ltd | 2021-07-12 | Diabetes Mellitus | Details | |
| Recombinant human insulin (HEC Pharm) | Approved | Yichang Hec Changjiang Pharmaceutical Co Ltd | Mainland China | Diabetes Mellitus | Yichang Hec Changjiang Pharmaceutical Co Ltd | 2020-06-05 | Diabetes Mellitus | Details | ||
| Isophane Protamine Recombinant Human Insulin Injection (Shenzhen Kexing Biotech) | Approved | Shenzhen Kexing Biological Engineering Co Ltd | 苏泌啉恩 | Mainland China | Diabetes Mellitus | Shenzhen Kexing Biological Engineering Co Ltd | 2003-02-24 | Diabetes Mellitus | Details | |
| Insulin glargine biosimilar (Tonghua Dongbao Pharmaceutical) | Approved | Tonghua Dongbao Pharmaceutical Co Ltd | 长舒霖 | Mainland China | Diabetes Mellitus | Tonghua Dongbao Pharmaceutical Co Ltd | 2019-12-06 | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Human insulin biosimilar (Square Pharmaceuticals) | Approved | Square Pharmaceuticals Ltd | Ansulin | Bangladesh | Diabetes Mellitus | Square Pharmaceuticals Ltd | 2013-01-01 | Diabetes Mellitus | Details | |
| Recombinant human insulin (United Laboratories International Holdings) | Approved | Zhongshan Branch of Zhuhai United Laboratories Co Ltd | 优思灵, Uslin, 优思灵R | Mainland China | Diabetes Mellitus | Zhongshan Branch of Zhuhai United Laboratories Co Ltd | 2009-11-25 | Diabetes Mellitus | Details | |
| Insulin glargine biosimilar (Getz Pharma) | Approved | Getz Pharma | Basagine | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Details | |||||
| Recombinant human insulin (Tonghua Dongbao Pharmaceutical) | Approved | Tonghua Dongbao Pharmaceutical Co Ltd | 甘舒霖, 甘舒霖R | Mainland China | Diabetes Mellitus | Tonghua Dongbao Pharmaceutical Co Ltd | 1998-01-01 | Diabetes Mellitus | Details | |
| Insulin aspart | NN-1218; INA-X14; Insulin X14 | Approved | Novo Nordisk A/S | Novolog, NovoRapid, NovoLog FlexPen, NovoMix, Fiasp, 诺和锐, NovoRapid/NovoLog | EU | Diabetes Mellitus | Novo Nordisk A/S | 1999-09-07 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Atherosclerosis; Delirium; Hypercholesterolemia; Hypertension; Postoperative Cognitive Complications; Coronary Disease; Cognitive Dysfunction; Alzheimer Disease; Cardiovascular Diseases; Diabetes Mellitus | Details |
| Insulin glargine biosimilar (LG Life Sciences) | Approved | Lg Life Sciences Ltd | Basugine | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Details | |||||
| Insulin biosimilar (Wockhardt) | Approved | Wockhardt Ltd | Wosulin Pen Royale, Wosulin, Wosulin®-R, Wosulin Pen | India | Diabetes Mellitus, Type 1 | Wockhardt Ltd | 2003-10-01 | Diabetes Mellitus, Type 1; Diabetes Mellitus | Details | |
| Insulin detemir | NN-304 | Approved | Novo Nordisk A/S | 诺和平, Levemir | EU | Diabetes Mellitus | Novo Nordisk A/S | 2004-06-01 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Atherosclerosis; Hypercholesterolemia; Hypertension; Diabetes, Gestational; Coronary Disease; Alzheimer Disease; Cognitive Dysfunction; Cardiovascular Diseases; Diabetes Mellitus | Details |
| Insulin biosimilar (Popular Pharmaceuticals) | Approved | Popular Pharmaceuticals | Insul R | Bangladesh | Diabetes Mellitus | Popular Pharmaceuticals | 2013-01-01 | Diabetes Mellitus | Details | |
| Insulin degludec/Liraglutide | NN-9068 | Approved | Novo Nordisk A/S | Xultophy, iDegLira | EU | Diabetes Mellitus, Type 2 | Novo Nordisk A/S | 2014-09-18 | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Imeglimin hydrochloride | EMD-387008; PXL-008 | Approved | Merck KGaA, Darmstadt, Germany | Twymeeg, ツイミーグ | Japan | Diabetes Mellitus, Type 2 | Poxel Sa, Sumitomo Dainippon Pharma Co Ltd | 2021-06-23 | Diabetes Mellitus, Type 2; Heart Diseases; Hepatic Insufficiency | Details |
| Insulin biosimilar (Sedico) | Approved | Sedico | Insulin H bio R | Egypt | Diabetes Mellitus | Sedico | 2013-02-01 | Diabetes Mellitus | Details | |
| Insulin aspart biosimilar (Biocon/Mylan) | MYL-1601D | Approved | Biocon Ltd | EU | Diabetes Mellitus | Biosimilar Collaborations Ireland Ltd | 2021-02-05 | Diabetes Mellitus | Details | |
| Insulin human (rDNA origin, Lilly) | U-500R; S-3300; LY-041001 | Approved | Eli Lilly And Company | Humulin R Kwikpen, Humulin, Humulin R, 优泌淋R, Humulin R Pen | United States | Diabetes Mellitus, Type 2 | Eli Lilly And Company | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Brain Neoplasms; Alzheimer Disease; Cognitive Dysfunction; Intracranial Aneurysm; Diabetes Mellitus | Details | |
| Insulin glargine biosimilar (Wockhardt) | Approved | Wockhardt Bio Ag | Glaritus, Glaritus Cart, Glaritus DispoPen | India | Diabetes Mellitus; Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Wockhardt Ltd | 2013-01-01 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Insulin aspart biosimilar (Tonghua Dongbao Pharmaceutical) | Approved | Tonghua Dongbao Pharmaceutical Co Ltd | Mainland China | Diabetes Mellitus | Tonghua Dongbao Pharmaceutical Co Ltd | 2021-10-19 | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | ||
| Human insulin biosimilar (MJ Biopharm) | Approved | Mj Biopharm Pvt | Biosulin | India | Diabetes Mellitus | Mj Biopharm Pvt | 2005-12-01 | Diabetes Mellitus | Details | |
| Insulin glargine biosimilar(Shandong New Time Pharmaceutical) | Approved | Lunan Pharmaceutical Group Co | Mainland China | Diabetes Mellitus | Shandong New Time Pharmaceutical Co Ltd | 2022-05-10 | Diabetes Mellitus | Details | ||
| Insulin lispro biosimilar (Sanofi) | SAR-342434 | Approved | Sanofi | Admelog | EU | Diabetes Mellitus | Sanofi Winthrop Industrie SA | 2017-07-18 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Insulin biosimilars (Bioton) | Approved | Bioton Sa | Mainland China | Diabetes Mellitus | Bioton Sa | 2013-01-01 | Diabetes Mellitus | Details | ||
| Insulin glargine biosimilar (Biocon) | MYL-1501D | Approved | Fujifilm Corp, Biocon Biopharmaceuticals, Mylan Nv | Semglee, Basalog | India | Diabetes Mellitus | null | 2009-01-01 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Insulin glargine biosimilar (Wanbang Biochemical) | Approved | Wanbang Biopharmaceuticals Co Ltd | Mainland China | Diabetes Mellitus | Wanbang Biopharmaceuticals Co Ltd | 2022-08-30 | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | ||
| Recombinant insulin glargine (Gan & Lee) | GL-GLA | Approved | Gan & Lee Biotech | 长秀霖, Basalin | Mainland China | Diabetes Mellitus | Gan & Lee Pharmaceuticals | 2005-05-10 | Diabetes Mellitus, Type 1; Diabetes Mellitus | Details |
| Insulin degludec/Insulin aspart | NN-1045; NN-5401; NN-1250/Insulin aspart | Approved | Novo Nordisk A/S | Ryzodeg, 诺和佳 | Japan | Diabetes Mellitus; Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Novo Nordisk A/S | 2012-12-25 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Insulin human (rDNA origin, Novo Nordisk) | NN-729 | Approved | Novo Nordisk A/S | Novolin R, 诺和灵R, Mixtard, Actraphane, Insulatard | United States | Diabetes Mellitus, Type 2 | Novo Nordisk Inc | 1991-06-25 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Smoking Cessation; Hyperglycemia; Alcoholism; Parkinson Disease; Alzheimer Disease; Cognitive Dysfunction; Pulmonary Disease, Chronic Obstructive; Lymphoma; Diabetes Mellitus | Details |
| Ceritinib | LDK-378; NVP-LDK378; NVP-LDK378-NX | Approved | Novartis Pharma Ag | 赞可达, Zykadia | EU | Carcinoma, Non-Small-Cell Lung | Novartis Europharm Ltd | 2014-04-29 | Colorectal Neoplasms; Melanoma; Adenocarcinoma; Carcinoma, Non-Small-Cell Lung; Thyroid Neoplasms; Brain metastases; Esophageal adenocarcinoma; Granuloma, Plasma Cell; Lymphoma, Large-Cell, Anaplastic; Hematologic Neoplasms; Hepatic Insufficiency; Cholangiocarcinoma; Pancreatic Neoplasms; Thyroid Carcinoma, Anaplastic; Neoplasms; Glioblastoma; Stomach Neoplasms | Details |
| Isophane Protamine Recombinant Human Insulin biosimilar (Popular Pharmaceuticals) | Approved | Popular Pharmaceuticals | Insulin N | Bangladesh | Diabetes Mellitus | Popular Pharmaceuticals | 2013-01-01 | Diabetes Mellitus | Details | |
| Insulin glargine | U-300; Gla-100; Gla-300; Hoe-901; Hoe-71GT; HOE901-U300 | Approved | Sanofi | 来得时, Lantus, Toujeo, Lantus XR, Optisulin | United States | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Sanofi-Aventis U.S. Llc | 2000-04-20 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Atherosclerosis; Diabetic Ketoacidosis; Hyperglycemia; Hypercholesterolemia; Hypertension; Coronary Disease; Cardiovascular Diseases; Diabetes Mellitus | Details |
| Insulin human (inhalation powder) | SAR-439065 | Approved | Mannkind Corporation | Technosphere Insulin System, Afrezza, Afresa | United States | Diabetes Mellitus, Type 2 | Mannkind Corporation | 2014-06-27 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Hyperglycemia; Asthma; Pulmonary Disease, Chronic Obstructive; Diabetes Mellitus | Details |
| Insulin human | HR-1799 | Approved | Sanofi Deutschland | Insulin Human Winthrop | EU | Diabetes Mellitus; Diabetic Coma; Diabetic Ketoacidosis | Sanofi-Aventis Deutschland Gmbh | 1997-02-21 | Diabetic Coma; Diabetic Ketoacidosis; Glaucoma; Diabetes Mellitus | Details |
| Insulin glargine biosimilar (Incepta Pharmaceuticals) | Approved | Incepta Pharmaceuticals Ltd | Bangladesh | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Incepta Pharmaceuticals Ltd | 2012-08-01 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Details | ||
| Insulin aspart biosimilar (Sanofi) | SAR-341402; SAR-Asp | Approved | Sanofi | Truvelog | EU | Diabetes Mellitus | Sanofi Winthrop Industrie SA | 2020-06-25 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Recombinant lispro insulin biosimilar (Gan & Lee) | Approved | Gan & Lee Pharmaceuticals | Prandilin, 速秀霖 | Mainland China | Diabetes Mellitus | Gan & Lee Pharmaceuticals | 2006-01-19 | Diabetes Mellitus | Details | |
| Rinsulin NPH (Geropharm) | Approved | Geropharm | Rinsulin NPH | Russian Federation | Diabetes Mellitus | Geropharm | 2012-10-01 | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Insulin glargine biosimilar (Lilly/Boehringer Ingelheim) | LY-2963016; LY2963016-U-100; LY2963016-U-200 | Approved | Boehringer Ingelheim Gmbh, Eli Lilly And Company | Basaglar, Abasaglar, Rezvoglar, Abasria | EU | Diabetes Mellitus | Eli Lilly Nederland BV | 2014-09-09 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Hyperglycemia; Renal Insufficiency, Chronic; Diabetes Mellitus | Details |
| Oral insulin (Generex Biotechnology) | Approved | Generex Biotechnology Corp | Oralin, Oralgen, Oral-lyn, Recosulin | Ecuador | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | null | 2005-01-01 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Details | |
| Insul 30/70 biosimilar (Popular Pharmaceuticals) | Approved | Popular Pharmaceuticals | Insul 30-70 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes, Gestational | Details | |||||
| Recombinant Human Insulin (Wanbang Pharma) | Approved | Wanbang Biopharmaceuticals Co Ltd | Mainland China | Diabetes Mellitus | Wanbang Biopharmaceuticals Co Ltd | 2003-08-29 | Diabetes Mellitus | Details | ||
| Insulin degludec | NN-1250; Insulin-454 | Approved | Novo Nordisk A/S | 诺和达, Tresiba | Japan | Diabetes Mellitus, Type 2; Diabetes Mellitus; Diabetes Mellitus, Type 1 | Novo Nordisk A/S | 2012-09-28 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Insulin glulisine | HMR-1964 | Approved | Sanofi | 艾倍得, Apidra | United States | Diabetes Mellitus | Sanofi-Aventis U.S. Llc | 2004-04-16 | Diabetes Mellitus, Type 1; Leukemia; Diabetes Mellitus, Type 2; Heart Failure; Myocardial Infarction; Down Syndrome; Hyperglycemia; Cardiovascular Diseases; Cognitive Dysfunction; Alzheimer Disease; Diabetes Mellitus; Respiratory Insufficiency | Details |
| Insulin biosimilar (Julphar) | Approved | Julphar (Gulf Pharmaceutical Industries) | Julphar Insulin R | Tunisia | Diabetes Mellitus | Julphar (Gulf Pharmaceutical Industries) | 2013-10-01 | Diabetes Mellitus | Details | |
| Insulin lispro protamine(25R) (Lilly ) | Approved | Lilly Suzhou Pharmaceutical Co Ltd | Diabetes Mellitus | Details | ||||||
| Insulin Aspart 50(Tonghua Dongbao Pharmaceutical) | Approved | Tonghua Dongbao Pharmaceutical Co Ltd | Mainland China | Diabetes Mellitus | Tonghua Dongbao Pharmaceutical Co Ltd | 2022-11-08 | Diabetes Mellitus | Details | ||
| Insulin Aspart 30(onghua Dongbao Pharmaceutical) | Approved | Tonghua Dongbao Pharmaceutical Co Ltd | Mainland China | Diabetes Mellitus | Tonghua Dongbao Pharmaceutical Co Ltd | 2022-11-08 | Diabetes Mellitus | Details | ||
| Recombinant insulin aspart biosimilar (Yichang Hec Changjiang Pharmaceutical) | Approved | Yichang Hec Changjiang Pharmaceutical Co Ltd | Mainland China | Diabetes Mellitus | Yichang Hec Changjiang Pharmaceutical Co Ltd | 2022-10-11 | Diabetes Mellitus | Details | ||
| Insulin Aspart 30 | Approved | Novo Nordisk A/S | 诺和锐30 | Mainland China | Diabetes Mellitus | Novo Nordisk A/S | 2009-09-30 | Diabetes Mellitus | Details | |
| Isophane Protamine Human Insulin(30R)(Gan & Lee ) | Approved | Gan & Lee Pharmaceuticals | Mainland China | Diabetes Mellitus | Gan & Lee Pharmaceuticals | 2021-05-19 | Diabetes Mellitus | Details | ||
| Isophane Protamine Human Insulin(50R)(Zhuhai United Laboratories ) | Approved | Zhongshan Branch of Zhuhai United Laboratories Co Ltd | 诺和灵50R | Mainland China | Diabetes Mellitus | Zhongshan Branch of Zhuhai United Laboratories Co Ltd | 2010-12-31 | Diabetes Mellitus | Details | |
| Insulin human (rDNA) biosimilar (Baxter) | Approved | Baxter International Inc | Inpremzia, Myxredlin | United States | Diabetes Mellitus | Baxter Healthcare Corp | 2019-06-20 | Diabetes Mellitus | Details | |
| Insulin aspart biosimilar (Hisun Pharma) | Approved | Zhejiang Hisun Pharmaceutical Co Ltd | Mainland China | Diabetes Mellitus | Zhejiang Hisun Pharmaceutical Co Ltd | 2021-09-13 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | ||
| Isophane Protamine Human Insulin(30R)(Hec Pharm) | Approved | Hec Pharm Co Ltd | 诺和灵30R | Mainland China | Diabetes Mellitus | Hec Pharm Co Ltd | 2023-09-19 | Diabetes Mellitus | Details | |
| Insulin Aspart 30 biosimilar (Jilin Huisheng Biological) | Approved | Jilin Huisheng Biopharmaceutical Co Ltd | Mainland China | Diabetes Mellitus | Jilin Huisheng Biopharmaceutical Co Ltd | 2023-12-26 | Diabetes Mellitus | Details | ||
| Insulin aspart biosimilar(Jilin Huisheng Biological) | Approved | Jilin Huisheng Biopharmaceutical Co Ltd | Mainland China | Diabetes Mellitus | Jilin Huisheng Biopharmaceutical Co Ltd | 2023-12-26 | Diabetes Mellitus | Details | ||
| Isophane Protamine Human Insulin(50R)(Novo Nordisk ) | Approved | Novo Nordisk A/S | 诺和灵50R | Mainland China | Diabetes Mellitus | Novo Nordisk (China)Pharmaceuticals Co Ltd | 2019-11-27 | Diabetes Mellitus | Details | |
| Isophane Protamine Human Insulin(30R)(Novo Nordisk ) | Approved | Novo Nordisk A/S | 诺和灵30R | Mainland China | Diabetes Mellitus | Novo Nordisk A/S | 1999-07-28 | Diabetes Mellitus | Details | |
| Insulin Aspart 50 biosimilar (Jilin Huisheng Biological) | Approved | Jilin Huisheng Biopharmaceutical Co Ltd | Mainland China | Diabetes Mellitus | Jilin Huisheng Biopharmaceutical Co Ltd | 2023-12-26 | Diabetes Mellitus | Details | ||
| Insulin biosimilar (Harvest Moon Pharmaceuticals) | Approved | Harvest Moon | Diabetes Mellitus | Details | ||||||
| 30/70 Mixture Recombinant Human Insulin(Tonghua Dongbao Pharmaceutical) | Approved | Tonghua Dongbao Pharmaceutical Co Ltd | 甘舒霖30R | Mainland China | Diabetes Mellitus | Tonghua Dongbao Pharmaceutical Co Ltd | 2002-05-18 | Diabetes Mellitus | Details | |
| Mixed Protamine Human Insulin(40R)(Tonghua Dongbao Pharmaceutical) | Approved | Tonghua Dongbao Pharmaceutical Co Ltd | 甘舒霖N | Mainland China | Diabetes Mellitus | Tonghua Dongbao Pharmaceutical Co Ltd | 1999-01-01 | Diabetes Mellitus | Details | |
| 50/50 Mixture Recombinant Human Insulin(Tonghua Dongbao Pharmaceutical) | Approved | Tonghua Dongbao Pharmaceutical Co Ltd | 甘舒霖50R | Mainland China | Diabetes Mellitus | Tonghua Dongbao Pharmaceutical Co Ltd | 2008-02-04 | Diabetes Mellitus | Details | |
| Insulin Aspart 50 | Approved | Novo Nordisk A/S | 诺和锐50 | Mainland China | Diabetes Mellitus | Novo Nordisk A/S | 2012-02-10 | Diabetes Mellitus | Details | |
| Insulin aspart biosimilar (Gan & Lee) | GL-ASP | Approved | Gan & Lee Biotech | Rapilin®30, Rapilin30, 锐秀霖30 | Mainland China | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Gan & Lee Biotech | 2020-06-05 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Isophane Protamine Human Insulin(30R)(Lilly) | Approved | Eli Lilly And Company | Mainland China | Diabetes Mellitus | Eli Lilly And Company | Diabetes Mellitus | Details | |||
| Insulin lispro protamine biosimilar(25R) (Gan & Lee) | Approved | Gan & Lee Biotech | Prandilin25, 速秀霖25 | Mainland China | Diabetes Mellitus | Gan & Lee Pharmaceuticals | 2014-05-09 | Diabetes Mellitus | Details | |
| Insulin lispro | LY-900027; LY-275585; Lys-B28; Pro-B29; LYSPRO; LY-900014 | Approved | Eli Lilly And Company | Humalog, 优泌乐, Eglucent, Liprolog, Lyumjev, Liumjev | EU | Diabetes Mellitus | Eli Lilly Nederland BV | 1996-04-30 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Insulin Resistance; Diabetic Neuropathies; Diabetes Mellitus; Hyperlipidemias | Details |
| Recombinant human insulin (Biocon) | Approved | Biocon Ltd | Insugen | India | Diabetes Mellitus | Biocon Ltd | 2004-11-10 | Diabetes Mellitus | Details | |
| Isophane Protamine Human Insulin(40R)(Tonghua Dongbao) | Approved | Tonghua Dongbao Pharmaceutical Co Ltd | Diabetes Mellitus | Details | ||||||
| Insulin glargine biosimilar (Zhuhai United) | Approved | Zhuhai United Laboratories Co Ltd | Mainland China | Diabetes Mellitus | Zhuhai United Laboratories Co Ltd | 2016-12-23 | Diabetes Mellitus | Details | ||
| Isophane Insulin(Polish Society of Diabetology) | Approved | Polish Society of Diabetology | Diabetes Mellitus | Details | ||||||
| Insulin human(Organon France) | Approved | Organon France | EU | Diabetes Mellitus | Organon France | 1999-11-03 | Diabetes Mellitus | Details | ||
| Isophane Protamine Recombinant Human Insulin(Novo Nordisk) | Approved | Novo Nordisk (China)Pharmaceuticals Co Ltd | 诺和灵N | Mainland China | Diabetes Mellitus | Novo Nordisk (China)Pharmaceuticals Co Ltd | 2016-06-16 | Diabetes Mellitus | Details | |
| Isophane Protamine Recombinant Human Insulin (BIOTON) | Approved | Bioton Sa | Mainland China | Diabetes Mellitus | Bioton Sa | 2013-03-29 | Diabetes Mellitus | Details | ||
| Insulin Glargine biosimilar (Liaoning Bo'Ao Bio-Pharmaceutical) | Approved | Liaoning Bo'Ao Bio-Pharmaceutical Co Ltd | Mainland China | Diabetes Mellitus | Liaoning Bo'Ao Bio-Pharmaceutical Co Ltd | 2023-12-05 | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | ||
| Insulin Aspart 30 (Yichang Hec Changjiang Pharmaceutical) | Approved | Yichang Hec Changjiang Pharmaceutical Co Ltd | Mainland China | Diabetes Mellitus | Yichang Hec Changjiang Pharmaceutical Co Ltd | 2022-11-01 | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | ||
| Isophane Protamine Recombinant Human Insulin (Hefei Tianmai Biotechnology) | Approved | Hefei Tianmai Biotechnology Development Co Ltd | Mainland China | Diabetes Mellitus | Hefei Tianmai Biotechnology Development Co Ltd | 2019-09-05 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | ||
| Recombinant human insulin (Hefei Tianmai Biotechnology) | Approved | Hefei Tianmai Biotechnology Development Co Ltd | Mainland China | Diabetes Mellitus | Hefei Tianmai Biotechnology Development Co Ltd | 2018-04-28 | Diabetes Mellitus | Details | ||
| Lixisenatide/Insulin glargine | HOE-901/AVE-0010 | Approved | Sanofi | Soliqua, iGlarLixi, Suliqua | United States | Diabetes Mellitus, Type 2 | Sanofi-Aventis U.S. Llc | 2016-11-21 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Details |
| Insulin icodec | 148-0287-A; LAI-287; Long-acting basal insulin analogue; NN-1436; Insulin-287; NNC-0148-0000-0287; NNC0148-0287 C; OI-287GT; NN-1956 | Approved | Novo Nordisk A/S | AWIQLI, Awiqli | Canada | Diabetes Mellitus | Novo Nordisk Canada Inc | 2024-03-12 | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Insulin lispro biosimilar (Wanbang Biopharma) | Approved | Wanbang Biopharmaceuticals Co Ltd | Mainland China | Diabetes Mellitus | Wanbang Biopharmaceuticals Co Ltd | 2022-01-25 | Diabetes Mellitus | Details | ||
| Recombinant human insulin (Shenzhen Kexing Biotech) | Approved | Shenzhen Kexing Biological Engineering Co Ltd | 苏泌啉, Su Bi Lin | Mainland China | Diabetes Mellitus | Shenzhen Kexing Biological Engineering Co Ltd | 2002-06-10 | Diabetes Mellitus | Details | |
| Recombinant insulin (Horizon Pharma/Ibatech) | Approved | Savient Pharmaceuticals Inc | Poland | Diabetes Mellitus, Type 1 | null | 2002-07-01 | Diabetes Mellitus, Type 1 | Details | ||
| Rosinsulin | Approved | Medsintez | Diabetes Mellitus | Details | ||||||
| Isophane Insulin | Approved | Wanbang Biopharmaceuticals Co Ltd | 万苏林 | Mainland China | Diabetes Mellitus | Wanbang Biopharmaceuticals Co Ltd | 1997-01-01 | Diabetes Mellitus | Details | |
| Recombinant human insulin (Incepta) | Approved | Incepta Pharmaceuticals Ltd | Maxsulin | Bangladesh | Diabetes Mellitus | Incepta Pharmaceuticals Ltd | 2012-01-01 | Diabetes Mellitus | Details | |
| Recombinant human insulin (SciGen) | Approved | Generex Biotechnology Corp | Recojet | India | Diabetes Mellitus | null | 2004-12-01 | Diabetes Mellitus | Details | |
| Insulin aspart biosimilar (Zhuhai United Laboratories) | Approved | Zhuhai United Laboratories Co Ltd | 联邦优倍灵, Ublin | Mainland China | Diabetes Mellitus | Zhuhai United Laboratories Co Ltd | 2021-07-12 | Diabetes Mellitus | Details | |
| Recombinant human insulin (HEC Pharm) | Approved | Yichang Hec Changjiang Pharmaceutical Co Ltd | Mainland China | Diabetes Mellitus | Yichang Hec Changjiang Pharmaceutical Co Ltd | 2020-06-05 | Diabetes Mellitus | Details |
| English Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Recombinant human insulin biosimilar (Rechon Life Sciences) | Phase 3 Clinical | Rechon Life Science Ab | Diabetes Mellitus, Type 1 | Details | |
| Recombinant human insulin (Nutrinia) | NTRA-9620 | Phase 3 Clinical | Nutrinia Inc | Diabetes Mellitus, Type 2 | Details |
| Recombinant insulin lisargine(Hefei Tianmai Biotech) | Phase 3 Clinical | Hefei Tianmai Biotechnology Development Co Ltd | Diabetes Mellitus | Details | |
| Insulin aspart biosimilar (Geropharm) | GP-40071; GP-40081 | Phase 3 Clinical | Geropharm | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Ersodetug | XPA.15.247; XOMA-358; XOMA-247; RZ358; RZ-358 | Phase 3 Clinical | Xoma Corp | Congenital Hyperinsulinism; Hypoglycemia | Details |
| Insulin mouth rinse (EastGate Biotech) | Phase 3 Clinical | Genome Pharmaceuticals, Eastgate Biotech | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Alzheimer Disease | Details | |
| Long-acting basal insulin analogue | LAI-287; NN-1436; NN-1956; OI-287GT; 148-0287-A; Insulin-287; NNC0148-0287 C; NNC-0148-0000-0287; Long-acting-insulin-287; Long-acting basal insulin analogue | Phase 3 Clinical | Novo Nordisk A/S | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Ultra fast-acting insulin (Adocia) | THDB-0206; THDB0206; BC-106-Insulin-Lispro; BC-222-Insulin-Lispro | Phase 3 Clinical | Adocia | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| INS-068 | INS-068 | Phase 3 Clinical | Jiangsu Hengrui Medicine Co Ltd, Chengdu Suncadia Medicine Co Ltd, Shanghai Hengrui Pharmaceutical Co Ltd | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Insulin glargine biosimilar (Geropharm) | Phase 3 Clinical | Geropharm | Diabetes Mellitus, Type 1; Diabetes Mellitus | Details | |
| Insulin biosimilar (Valin Technologies) | Van-101 | Phase 3 Clinical | Valin Technologies | Diabetes Mellitus | Details |
| Insulin degludec/Insulin aspart(HEC) | Phase 3 Clinical | Dongguan City Hec Biological Medicine Res And Development Co Ltd, Guangdong Dongyangguang Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin degludec biosimilar (Hec) | RD15003 | Phase 3 Clinical | Dongguan City Hec Biological Medicine Res And Development Co Ltd | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Insulin aspart biosimilar(Amphastar) | I-004 | Phase 3 Clinical | Amphastar Pharmaceuticals Inc | Details | |
| TOTUM•63 | TOTUM-63 | Phase 3 Clinical | Valbiotis | Diabetes Mellitus, Type 2 | Details |
| Recombinant human insulin(Elgan) | ELGN-2112; ELGN-GI | Phase 3 Clinical | Elgan Pharma Ltd | Short Bowel Syndrome; Malabsorption Syndromes; Premature Birth | Details |
| Insulin degludec biosimilar (Chia Tai-Tianqing) | Phase 3 Clinical | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin degludec biosimilar (Jilin Jinsheng) | Phase 3 Clinical | Jilin Jinsheng Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Insulin degludec biosimilar (Chenan Biopharmaceutical) | Phase 3 Clinical | Chongqing Chenan Biopharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin degludec biosimilar (Zhuhai United) | Phase 3 Clinical | Zhuhai United Laboratories Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin aspart biosimilar (Kunpeng) | Phase 3 Clinical | Ningbo Kunpeng Biological Technology Co Ltd | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Protamine Recombinant Human Insulin (Gan & Lee Pharmaceuticals) | Phase 3 Clinical | Gan & Lee Pharmaceuticals | Diabetes Mellitus, Type 2 | Details | |
| Insulin icodec/Semaglutide | Phase 3 Clinical | Novo Nordisk A/S | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Insulin degludec/Insulin aspart(Jilin Huisheng) | NN-1045; NN-5401; NN-1250/Insulin aspart | Phase 3 Clinical | Jilin Huisheng Biopharmaceutical Co Ltd | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Isophane Protamine Recombinant Human Insulin (Jilin Huisheng Biological ) | Phase 3 Clinical | Jilin Huisheng Biopharmaceutical Co Ltd | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Insulin aspart (Jilin Jinsheng) | Phase 3 Clinical | Jilin Jinsheng Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Isophane Protamine Recombinant Human Insulin (Pre-Mixed 30R) (Hec Pharm) | Phase 3 Clinical | Yichang Hec Changjiang Pharmaceutical Co Ltd | Diabetes Mellitus | Details | |
| Mixed Protamine Zinc Recombinant Insulin Lispro Injection(25R)(Tonghua Dongbao) | Phase 3 Clinical | Tonghua Dongbao Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Insulin enteric-coated(Tsinghua University) | Phase 3 Clinical | Tsinghua University, Beijing Representative Office Of Hong Kong Fushi Bioengineering Co Ltd | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Details | |
| Insulin Aspart (Beijing Sl Pharmaceutical) | Phase 3 Clinical | Beijing Sl Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Recombinant human insulin(30/70) (Zhejiang Hisun Pharmaceutical) | Phase 3 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Recombinant insulin glargine (Chongqing Fujin Biological Medicine) | Phase 3 Clinical | Chongqing Fujin Biological Medicine Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin Glargine (Zhejiang Hisun Pharmaceutical) | HS004 | Phase 3 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd | Diabetes Mellitus | Details |
| Linsitinib | OSI-906; ASP-7487; OSI-906AA | Phase 3 Clinical | Astellas Pharma Inc, National Cancer Institute | Multiple Myeloma; Paraganglioma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Carcinoma, Squamous Cell; Orbital Diseases; Eye Diseases; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Exophthalmos; Breast Neoplasms; Prostatic Neoplasms; Sarcoma, Ewing; Adrenocortical Carcinoma; Thyroid Diseases; Graves Ophthalmopathy; Endocrine System Diseases; Skin Neoplasms; Neoplasms; Small Cell Lung Carcinoma; Pancreatic Neoplasms; Hashimoto Disease; Head and Neck Neoplasms; Ovarian Neoplasms; Carney Complex; Liver Neoplasms; Solid tumours; Chondrosarcoma | Details |
| Recombinant insulin aspart biosimilar (Biogenomics) | Phase 3 Clinical | Biogenomics | Diabetes Mellitus, Type 2 | Details | |
| AKP-11 | AKP-11 | Phase 2 Clinical | Akaal Pharma | Psoriasis; Dermatitis, Atopic | Details |
| Pramlintide/Insulin | ADO-09 | Phase 2 Clinical | Adocia | Diabetes Mellitus, Type 1 | Details |
| Fast-acting human insulin (Adocia) | BioChaperone-222; Hinsbet-U500 | Phase 2 Clinical | Adocia | Diabetes Mellitus, Type 1; Metabolic Syndrome; Cognitive Dysfunction; Alzheimer Disease | Details |
| HDV Insulin (Diasome Pharmaceuticals) | Phase 2 Clinical | Eli Lilly And Company | Diabetes Mellitus, Type 1; Diabetes Mellitus | Details | |
| scp776 | scp776 | Phase 2 Clinical | Silver Creek Pharmaceuticals Inc | Stroke | Details |
| NT-219 | NT-219; NT219 | Phase 2 Clinical | TyrNovo Ltd | Solid tumours; Head and Neck Neoplasms; Squamous Cell Carcinoma of Head and Neck; Colorectal Neoplasms; Neoplasm Metastasis | Details |
| GZR-101 | GZR101 | Phase 2 Clinical | Gan & Lee Pharmaceuticals | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| GZR-4 | GZR4; GZR-4 | Phase 2 Clinical | Gan & Lee Pharmaceuticals | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| HR-17031 | HR17031 | Phase 2 Clinical | Jiangsu Hengrui Medicine Co Ltd | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Human insulin biosimilar(Indonesia University) | Phase 2 Clinical | Indonesia University | Diabetes Mellitus | Details | |
| Insulin oral (Diabetology) | DTY-001 | Phase 2 Clinical | Diabetology Ltd | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Details |
| Insulin lispro (Diasome Pharmaceuticals) | Phase 2 Clinical | Diasome Pharmaceuticals Inc | Diabetes Mellitus, Type 1 | Details | |
| Pegylated recombinant human insulin (Chongqing Fujin Biomedical) | CA001 | Phase 1 Clinical | Chongqing Fujin Biological Medicine Co Ltd | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Insulin detemir biosimilar (Tonghua Dongbao Pharmaceutical) | Phase 1 Clinical | Tonghua Dongbao Biotechnology Co Ltd | Diabetes Mellitus | Details | |
| Dulaglutide/Basal insulin Fc | Phase 1 Clinical | Eli Lilly And Company | Diabetes Mellitus | Details | |
| Basal insulin Fc (Eli Lilly) | Phase 1 Clinical | Eli Lilly And Company | Diabetes Mellitus | Details | |
| AB-101(Rezolute) | AB-101 | Phase 1 Clinical | Rezolute | Diabetes Mellitus, Type 1; Hepatitis B, Chronic | Details |
| Insulin biosimilar (University of Montreal Health Centre) | Phase 1 Clinical | University Of Montreal Health Centre | Glaucoma | Details | |
| NP-01 (Medesis Pharma SA) | NP01 | Phase 1 Clinical | Medesis Pharma Sa | Diabetes Mellitus, Type 2 | Details |
| Insulin (Coremed) | Phase 1 Clinical | Coremed | Diabetes Mellitus | Details | |
| Semaglutide/Long-acting basal insulin analogue | NNC0148-0287sema | Phase 1 Clinical | Novo Nordisk A/S | Diabetes Mellitus, Type 2 | Details |
| Insulin lispro/Insulin glargine | THDB-0207 | Phase 1 Clinical | Adocia | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Basal insulin acylated (Lilly) | Phase 1 Clinical | Eli Lilly And Company | Diabetes Mellitus | Details | |
| Insulin glargine biosimilar (Valin Technologies) | Phase 1 Clinical | Valin Technologies | Diabetes Mellitus | Details | |
| Insulin degludec biosimilar (Tonghua Dongbao) | Phase 1 Clinical | Tonghua Dongbao Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin glargine(GeneSys Biologics) | GEN-1501 | Phase 1 Clinical | GeneSys Biologics Pvt Ltd | Diabetes Mellitus | Details |
| Glucose sensing insulin receptor agonist therapeutic (Eli Lilly and Company) | Phase 1 Clinical | Eli Lilly And Company | Obesity; Diabetes Mellitus | Details | |
| Glucagon/insulin human(Abvance Therapeutics) | ABV-100 | Phase 1 Clinical | Abvance Therapeutics | Hypoglycemia | Details |
| Insulin degludec/Liraglutide(Dongyangguang) | Phase 1 Clinical | Dongguan City Hec Biological Medicine Res And Development Co Ltd, Guangdong Dongyangguang Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin degludec/Insulin aspart(Hangzhou Zhongmei Huadong) | Phase 1 Clinical | Hangzhou Zhongmei Huadong Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin degludec/Liraglutide(Huisheng Biopharmaceutical) | Phase 1 Clinical | Jilin Huisheng Biopharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin degludec/Insulin aspart (Chongqing Chenan Biopharma) | Phase 1 Clinical | Chongqing Chenan Biopharmaceutical Co Ltd | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Insulin human (Columbia University) | Phase 1 Clinical | Columbia University | Prediabetic State; Metabolic Dysfunction-Associated Steatotic Liver Disease; Insulin Resistance; Hyperinsulinism; Obesity | Details | |
| Insulin aspart biosimilar(Jiangsu HengRui) | INS-062; INS062 | Phase 1 Clinical | Jiangsu Hengrui Medicine Co Ltd | Diabetes Mellitus | Details |
| Insulin degludec/Liraglutide(Lianbang Biotech) | Phase 1 Clinical | Lianbang Biotechnology (Zhuhai Hengqin) Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin degludec biosimilar(Wanbang Pharma) | Phase 1 Clinical | Wanbang Biopharmaceuticals Co Ltd | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Insulin degludec biosimilar (Zhongmei Huadong) | Phase 1 Clinical | Hangzhou Zhongmei Huadong Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin degludec biosimilar (Nanjing Cellnuo) | Phase 1 Clinical | Nanjing Cellnuo Biopharmaceutical Co Ltd, Nanjing Sinor Biotechnology Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin aspart biosimilar(Jiangsu HengRui)/INS-068 | HR20014; HR-20014 | Phase 1 Clinical | Jiangsu Hengrui Medicine Co Ltd | Diabetes Mellitus | Details |
| BC Combo U200 | Phase 1 Clinical | Adocia, Tonghua Dongbao Pharmaceutical Co Ltd | Diabetes Mellitus | Details | |
| Insulin degludec biosimilar (Hisun Pharma) | Phase 1 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin degludec/Insulin aspart (United Laboratories) | Phase 1 Clinical | Lianbang Biotechnology (Zhuhai Hengqin) Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin degludec biosimilar(Zhuhai Jibaikang) | Phase 1 Clinical | Zhuhai Jibaikang Biological Tech Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin lispro protamine biosimilar(50R) (Gan & Lee) | Phase 1 Clinical | Gan & Lee Pharmaceuticals | Diabetes Mellitus | Details | |
| Insulin aspart(Chongqing Chenan) | Phase 1 Clinical | Chongqing Chenan Biopharmaceutical Co Ltd | Diabetes Mellitus | Details | |
| Mixed Protamine Zinc Recombinant Insulin Lispro Injection(50R/25R)(Fosun Pharma) | Phase 1 Clinical | Fosun Holdings Ltd | Diabetes Mellitus, Type 2 | Details | |
| HR-011408 | HR011408; HR-011408 | Phase 1 Clinical | Jiangsu Hengrui Medicine Co Ltd | Diabetes Mellitus | Details |
| Insulin degludec(Beijing ShuangLu Pharmaceutical) | Phase 1 Clinical | Beijing Sl Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Mixed Protamine Zinc Recombinant Insulin Lispro Injection(50R)(Tonghua Dongbao) | Phase 1 Clinical | Tonghua Dongbao Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Human insulin (Ningbo Kunpeng) | Phase 1 Clinical | Ningbo Kunpeng Biological Technology Co Ltd | Diabetes Mellitus | Details | |
| NNC0363-0845 | Phase 1 Clinical | Novo Nordisk A/S | Diabetes Mellitus, Type 1 | Details | |
| Ultra-concentrated rapid acting insulin aspart (Arecor) | AT-278; AT278 | Phase 1 Clinical | Arecor Ltd | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Details |
| Insulin Aspart 30 (Liaoning Bo'Ao Bio-Pharmaceutical) | Phase 1 Clinical | Liaoning Bo'Ao Bio-Pharmaceutical Co Ltd | Diabetes Mellitus | Details | |
| Recombinant human insulin (Zhejiang Hisun Pharmaceutical) | Phase 1 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| CB-4211 | CB-4211 | Phase 1 Clinical | Cohbar Inc | Metabolic Dysfunction-Associated Steatotic Liver Disease | Details |
| Recombinant human insulin (Eastgate Biotech) | EGP-1214 | Clinical | Eastgate Biotech | Prediabetic State; Diabetes Mellitus, Type 2 | Details |
| Oral insulin (Purede) | Clinical | Puredel | Diabetes Mellitus | Details | |
| Recombinant human insulin biosimilar (Rechon Life Sciences) | Phase 3 Clinical | Rechon Life Science Ab | Diabetes Mellitus, Type 1 | Details | |
| Recombinant human insulin (Nutrinia) | NTRA-9620 | Phase 3 Clinical | Nutrinia Inc | Diabetes Mellitus, Type 2 | Details |
| Recombinant insulin lisargine(Hefei Tianmai Biotech) | Phase 3 Clinical | Hefei Tianmai Biotechnology Development Co Ltd | Diabetes Mellitus | Details | |
| Insulin aspart biosimilar (Geropharm) | GP-40071; GP-40081 | Phase 3 Clinical | Geropharm | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Ersodetug | XPA.15.247; XOMA-358; XOMA-247; RZ358; RZ-358 | Phase 3 Clinical | Xoma Corp | Congenital Hyperinsulinism; Hypoglycemia | Details |
| Insulin mouth rinse (EastGate Biotech) | Phase 3 Clinical | Genome Pharmaceuticals, Eastgate Biotech | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Alzheimer Disease | Details | |
| Long-acting basal insulin analogue | LAI-287; NN-1436; NN-1956; OI-287GT; 148-0287-A; Insulin-287; NNC0148-0287 C; NNC-0148-0000-0287; Long-acting-insulin-287; Long-acting basal insulin analogue | Phase 3 Clinical | Novo Nordisk A/S | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Ultra fast-acting insulin (Adocia) | THDB-0206; THDB0206; BC-106-Insulin-Lispro; BC-222-Insulin-Lispro | Phase 3 Clinical | Adocia | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| INS-068 | INS-068 | Phase 3 Clinical | Jiangsu Hengrui Medicine Co Ltd, Chengdu Suncadia Medicine Co Ltd, Shanghai Hengrui Pharmaceutical Co Ltd | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Insulin glargine biosimilar (Geropharm) | Phase 3 Clinical | Geropharm | Diabetes Mellitus, Type 1; Diabetes Mellitus | Details | |
| Insulin biosimilar (Valin Technologies) | Van-101 | Phase 3 Clinical | Valin Technologies | Diabetes Mellitus | Details |
| Insulin degludec/Insulin aspart(HEC) | Phase 3 Clinical | Dongguan City Hec Biological Medicine Res And Development Co Ltd, Guangdong Dongyangguang Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin degludec biosimilar (Hec) | RD15003 | Phase 3 Clinical | Dongguan City Hec Biological Medicine Res And Development Co Ltd | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Insulin aspart biosimilar(Amphastar) | I-004 | Phase 3 Clinical | Amphastar Pharmaceuticals Inc | Details | |
| TOTUM•63 | TOTUM-63 | Phase 3 Clinical | Valbiotis | Diabetes Mellitus, Type 2 | Details |
| Recombinant human insulin(Elgan) | ELGN-2112; ELGN-GI | Phase 3 Clinical | Elgan Pharma Ltd | Short Bowel Syndrome; Malabsorption Syndromes; Premature Birth | Details |
| Insulin degludec biosimilar (Chia Tai-Tianqing) | Phase 3 Clinical | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin degludec biosimilar (Jilin Jinsheng) | Phase 3 Clinical | Jilin Jinsheng Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Insulin degludec biosimilar (Chenan Biopharmaceutical) | Phase 3 Clinical | Chongqing Chenan Biopharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin degludec biosimilar (Zhuhai United) | Phase 3 Clinical | Zhuhai United Laboratories Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin aspart biosimilar (Kunpeng) | Phase 3 Clinical | Ningbo Kunpeng Biological Technology Co Ltd | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Protamine Recombinant Human Insulin (Gan & Lee Pharmaceuticals) | Phase 3 Clinical | Gan & Lee Pharmaceuticals | Diabetes Mellitus, Type 2 | Details | |
| Insulin icodec/Semaglutide | Phase 3 Clinical | Novo Nordisk A/S | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Insulin degludec/Insulin aspart(Jilin Huisheng) | NN-1045; NN-5401; NN-1250/Insulin aspart | Phase 3 Clinical | Jilin Huisheng Biopharmaceutical Co Ltd | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Isophane Protamine Recombinant Human Insulin (Jilin Huisheng Biological ) | Phase 3 Clinical | Jilin Huisheng Biopharmaceutical Co Ltd | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Insulin aspart (Jilin Jinsheng) | Phase 3 Clinical | Jilin Jinsheng Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Isophane Protamine Recombinant Human Insulin (Pre-Mixed 30R) (Hec Pharm) | Phase 3 Clinical | Yichang Hec Changjiang Pharmaceutical Co Ltd | Diabetes Mellitus | Details | |
| Mixed Protamine Zinc Recombinant Insulin Lispro Injection(25R)(Tonghua Dongbao) | Phase 3 Clinical | Tonghua Dongbao Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Insulin enteric-coated(Tsinghua University) | Phase 3 Clinical | Tsinghua University, Beijing Representative Office Of Hong Kong Fushi Bioengineering Co Ltd | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Details | |
| Insulin Aspart (Beijing Sl Pharmaceutical) | Phase 3 Clinical | Beijing Sl Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Recombinant human insulin(30/70) (Zhejiang Hisun Pharmaceutical) | Phase 3 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Recombinant insulin glargine (Chongqing Fujin Biological Medicine) | Phase 3 Clinical | Chongqing Fujin Biological Medicine Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin Glargine (Zhejiang Hisun Pharmaceutical) | HS004 | Phase 3 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd | Diabetes Mellitus | Details |
| Linsitinib | OSI-906; ASP-7487; OSI-906AA | Phase 3 Clinical | Astellas Pharma Inc, National Cancer Institute | Multiple Myeloma; Paraganglioma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Carcinoma, Squamous Cell; Orbital Diseases; Eye Diseases; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Exophthalmos; Breast Neoplasms; Prostatic Neoplasms; Sarcoma, Ewing; Adrenocortical Carcinoma; Thyroid Diseases; Graves Ophthalmopathy; Endocrine System Diseases; Skin Neoplasms; Neoplasms; Small Cell Lung Carcinoma; Pancreatic Neoplasms; Hashimoto Disease; Head and Neck Neoplasms; Ovarian Neoplasms; Carney Complex; Liver Neoplasms; Solid tumours; Chondrosarcoma | Details |
| Recombinant insulin aspart biosimilar (Biogenomics) | Phase 3 Clinical | Biogenomics | Diabetes Mellitus, Type 2 | Details | |
| AKP-11 | AKP-11 | Phase 2 Clinical | Akaal Pharma | Psoriasis; Dermatitis, Atopic | Details |
| Pramlintide/Insulin | ADO-09 | Phase 2 Clinical | Adocia | Diabetes Mellitus, Type 1 | Details |
| Fast-acting human insulin (Adocia) | BioChaperone-222; Hinsbet-U500 | Phase 2 Clinical | Adocia | Diabetes Mellitus, Type 1; Metabolic Syndrome; Cognitive Dysfunction; Alzheimer Disease | Details |
| HDV Insulin (Diasome Pharmaceuticals) | Phase 2 Clinical | Eli Lilly And Company | Diabetes Mellitus, Type 1; Diabetes Mellitus | Details | |
| scp776 | scp776 | Phase 2 Clinical | Silver Creek Pharmaceuticals Inc | Stroke | Details |
| NT-219 | NT-219; NT219 | Phase 2 Clinical | TyrNovo Ltd | Solid tumours; Head and Neck Neoplasms; Squamous Cell Carcinoma of Head and Neck; Colorectal Neoplasms; Neoplasm Metastasis | Details |
| GZR-101 | GZR101 | Phase 2 Clinical | Gan & Lee Pharmaceuticals | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| GZR-4 | GZR4; GZR-4 | Phase 2 Clinical | Gan & Lee Pharmaceuticals | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| HR-17031 | HR17031 | Phase 2 Clinical | Jiangsu Hengrui Medicine Co Ltd | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Human insulin biosimilar(Indonesia University) | Phase 2 Clinical | Indonesia University | Diabetes Mellitus | Details | |
| Insulin oral (Diabetology) | DTY-001 | Phase 2 Clinical | Diabetology Ltd | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Details |
| Insulin lispro (Diasome Pharmaceuticals) | Phase 2 Clinical | Diasome Pharmaceuticals Inc | Diabetes Mellitus, Type 1 | Details | |
| Pegylated recombinant human insulin (Chongqing Fujin Biomedical) | CA001 | Phase 1 Clinical | Chongqing Fujin Biological Medicine Co Ltd | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Insulin detemir biosimilar (Tonghua Dongbao Pharmaceutical) | Phase 1 Clinical | Tonghua Dongbao Biotechnology Co Ltd | Diabetes Mellitus | Details | |
| Dulaglutide/Basal insulin Fc | Phase 1 Clinical | Eli Lilly And Company | Diabetes Mellitus | Details | |
| Basal insulin Fc (Eli Lilly) | Phase 1 Clinical | Eli Lilly And Company | Diabetes Mellitus | Details | |
| AB-101(Rezolute) | AB-101 | Phase 1 Clinical | Rezolute | Diabetes Mellitus, Type 1; Hepatitis B, Chronic | Details |
| Insulin biosimilar (University of Montreal Health Centre) | Phase 1 Clinical | University Of Montreal Health Centre | Glaucoma | Details | |
| NP-01 (Medesis Pharma SA) | NP01 | Phase 1 Clinical | Medesis Pharma Sa | Diabetes Mellitus, Type 2 | Details |
| Insulin (Coremed) | Phase 1 Clinical | Coremed | Diabetes Mellitus | Details | |
| Semaglutide/Long-acting basal insulin analogue | NNC0148-0287sema | Phase 1 Clinical | Novo Nordisk A/S | Diabetes Mellitus, Type 2 | Details |
| Insulin lispro/Insulin glargine | THDB-0207 | Phase 1 Clinical | Adocia | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Diabetes Mellitus | Details |
| Basal insulin acylated (Lilly) | Phase 1 Clinical | Eli Lilly And Company | Diabetes Mellitus | Details | |
| Insulin glargine biosimilar (Valin Technologies) | Phase 1 Clinical | Valin Technologies | Diabetes Mellitus | Details | |
| Insulin degludec biosimilar (Tonghua Dongbao) | Phase 1 Clinical | Tonghua Dongbao Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin glargine(GeneSys Biologics) | GEN-1501 | Phase 1 Clinical | GeneSys Biologics Pvt Ltd | Diabetes Mellitus | Details |
| Glucose sensing insulin receptor agonist therapeutic (Eli Lilly and Company) | Phase 1 Clinical | Eli Lilly And Company | Obesity; Diabetes Mellitus | Details | |
| Glucagon/insulin human(Abvance Therapeutics) | ABV-100 | Phase 1 Clinical | Abvance Therapeutics | Hypoglycemia | Details |
| Insulin degludec/Liraglutide(Dongyangguang) | Phase 1 Clinical | Dongguan City Hec Biological Medicine Res And Development Co Ltd, Guangdong Dongyangguang Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin degludec/Insulin aspart(Hangzhou Zhongmei Huadong) | Phase 1 Clinical | Hangzhou Zhongmei Huadong Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin degludec/Liraglutide(Huisheng Biopharmaceutical) | Phase 1 Clinical | Jilin Huisheng Biopharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin degludec/Insulin aspart (Chongqing Chenan Biopharma) | Phase 1 Clinical | Chongqing Chenan Biopharmaceutical Co Ltd | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Insulin human (Columbia University) | Phase 1 Clinical | Columbia University | Prediabetic State; Metabolic Dysfunction-Associated Steatotic Liver Disease; Insulin Resistance; Hyperinsulinism; Obesity | Details | |
| Insulin aspart biosimilar(Jiangsu HengRui) | INS-062; INS062 | Phase 1 Clinical | Jiangsu Hengrui Medicine Co Ltd | Diabetes Mellitus | Details |
| Insulin degludec/Liraglutide(Lianbang Biotech) | Phase 1 Clinical | Lianbang Biotechnology (Zhuhai Hengqin) Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin degludec biosimilar(Wanbang Pharma) | Phase 1 Clinical | Wanbang Biopharmaceuticals Co Ltd | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Insulin degludec biosimilar (Zhongmei Huadong) | Phase 1 Clinical | Hangzhou Zhongmei Huadong Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin degludec biosimilar (Nanjing Cellnuo) | Phase 1 Clinical | Nanjing Cellnuo Biopharmaceutical Co Ltd, Nanjing Sinor Biotechnology Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin aspart biosimilar(Jiangsu HengRui)/INS-068 | HR20014; HR-20014 | Phase 1 Clinical | Jiangsu Hengrui Medicine Co Ltd | Diabetes Mellitus | Details |
| BC Combo U200 | Phase 1 Clinical | Adocia, Tonghua Dongbao Pharmaceutical Co Ltd | Diabetes Mellitus | Details | |
| Insulin degludec biosimilar (Hisun Pharma) | Phase 1 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin degludec/Insulin aspart (United Laboratories) | Phase 1 Clinical | Lianbang Biotechnology (Zhuhai Hengqin) Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin degludec biosimilar(Zhuhai Jibaikang) | Phase 1 Clinical | Zhuhai Jibaikang Biological Tech Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Insulin lispro protamine biosimilar(50R) (Gan & Lee) | Phase 1 Clinical | Gan & Lee Pharmaceuticals | Diabetes Mellitus | Details | |
| Insulin aspart(Chongqing Chenan) | Phase 1 Clinical | Chongqing Chenan Biopharmaceutical Co Ltd | Diabetes Mellitus | Details | |
| Mixed Protamine Zinc Recombinant Insulin Lispro Injection(50R/25R)(Fosun Pharma) | Phase 1 Clinical | Fosun Holdings Ltd | Diabetes Mellitus, Type 2 | Details | |
| HR-011408 | HR011408; HR-011408 | Phase 1 Clinical | Jiangsu Hengrui Medicine Co Ltd | Diabetes Mellitus | Details |
| Insulin degludec(Beijing ShuangLu Pharmaceutical) | Phase 1 Clinical | Beijing Sl Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| Mixed Protamine Zinc Recombinant Insulin Lispro Injection(50R)(Tonghua Dongbao) | Phase 1 Clinical | Tonghua Dongbao Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2; Diabetes Mellitus | Details | |
| Human insulin (Ningbo Kunpeng) | Phase 1 Clinical | Ningbo Kunpeng Biological Technology Co Ltd | Diabetes Mellitus | Details | |
| NNC0363-0845 | Phase 1 Clinical | Novo Nordisk A/S | Diabetes Mellitus, Type 1 | Details | |
| Ultra-concentrated rapid acting insulin aspart (Arecor) | AT-278; AT278 | Phase 1 Clinical | Arecor Ltd | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2 | Details |
| Insulin Aspart 30 (Liaoning Bo'Ao Bio-Pharmaceutical) | Phase 1 Clinical | Liaoning Bo'Ao Bio-Pharmaceutical Co Ltd | Diabetes Mellitus | Details | |
| Recombinant human insulin (Zhejiang Hisun Pharmaceutical) | Phase 1 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2 | Details | |
| CB-4211 | CB-4211 | Phase 1 Clinical | Cohbar Inc | Metabolic Dysfunction-Associated Steatotic Liver Disease | Details |
| Recombinant human insulin (Eastgate Biotech) | EGP-1214 | Clinical | Eastgate Biotech | Prediabetic State; Diabetes Mellitus, Type 2 | Details |
| Oral insulin (Purede) | Clinical | Puredel | Diabetes Mellitus | Details |
This web search service is supported by Google Inc.







