Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
Multiple myeloma (MM) is the second-largest malignant tumor in hematology. It originates from the malignant proliferation of plasma cells in the hematopoietic tissue of the bone marrow and ultimately leads to organ or tissue damage. The disease usually occurs in elderly patients and the main clinical manifestations are damaged skeletal system, kidney damage, and blood system damage.
In terms of market scale, in 2018, the global market for MM-related therapeutic drugs totaled approximately $19.4 billion. Frost & Sullivan predicts that the market scale of MM-related therapeutic drugs is expected to further grow to $40 billion by 2025.
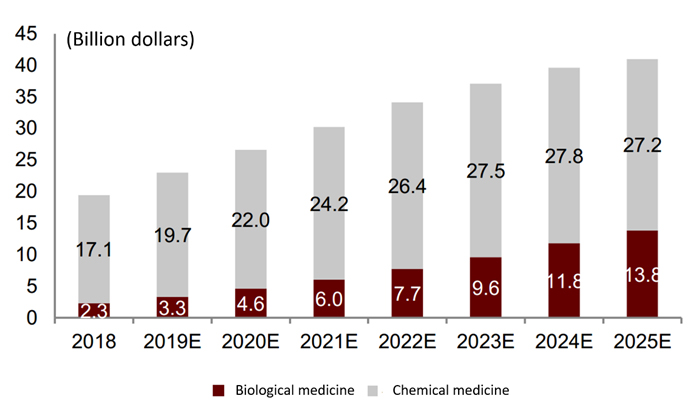
The history and forecast market scale of MM therapy
In the past ten years, the treatment of MM has mostly been based on comprehensive treatment, including chemotherapy, radiotherapy, and autologous stem cell transplantation.
1. Chemotherapy, including traditional chemotherapy drugs (such as melphalan, adriamycin, and cyclophosphamide, etc.), glucocorticoids (such as dexamethasone, prednisone, etc.), immunomodulators (such as thalidomide, lenalidomide), Pomalidomide, etc.) and protease inhibitors (such as bortezomib, carfilzomib, ixazomib, pabirestat, etc.).
2. Radiotherapy for patients with localized myeloma, local bone pain, and symptoms of spinal cord compression.
3. Autologous stem cell transplantation directly eliminates myeloma cells by destroying the tumor microenvironment and promotes the anti-myeloma response mediated by T cells.
Although the patient's condition has improved, the disease still inevitably recurs. Based on the characteristics of MM that are difficult to cure and easy to drug-resistant relapse, the current treatment methods are far from meeting the current medical needs. How to change the poor survival status of patients with refractory relapsed MM and improve the life quality of patients is still a problem that needs to be solved urgently.
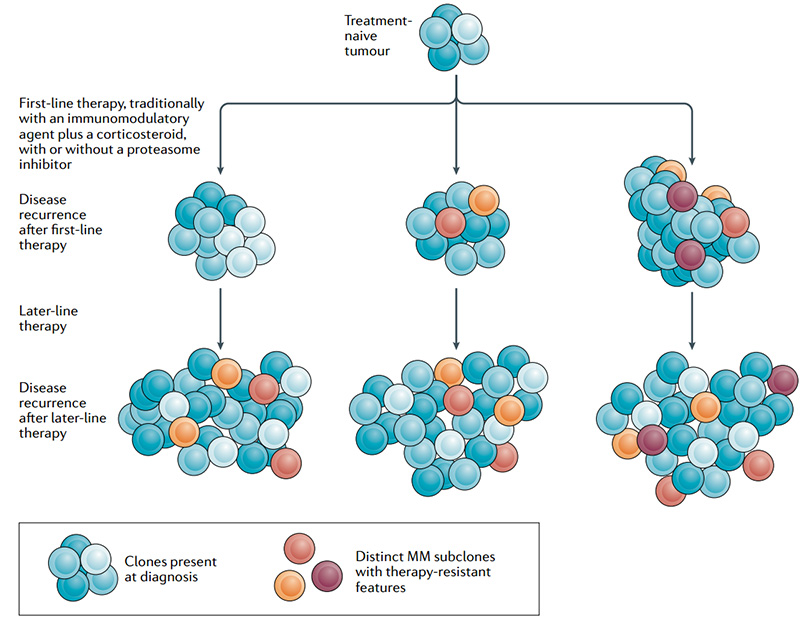
Heterogeneity and drug resistance of MM subclones
The successful development and application of macromolecular targeted therapy drugs have significantly improved the progression free survival (PFS) and overall survival (OS) of relapsed/refractory patients, and rapidly increased from fourth-line therapy to first-line therapy. To develop drugs specifically targeting MM, it is necessary to find the target antigen specifically expressed on the surface of malignant plasma cells. Studies have found that targets such as CD38, CD138, CD47, SLAMF7, BCMA and GPRC5D are highly specifically expressed on the surface of malignant plasma cells, which undoubtedly become potential targets for MM immunotherapy.
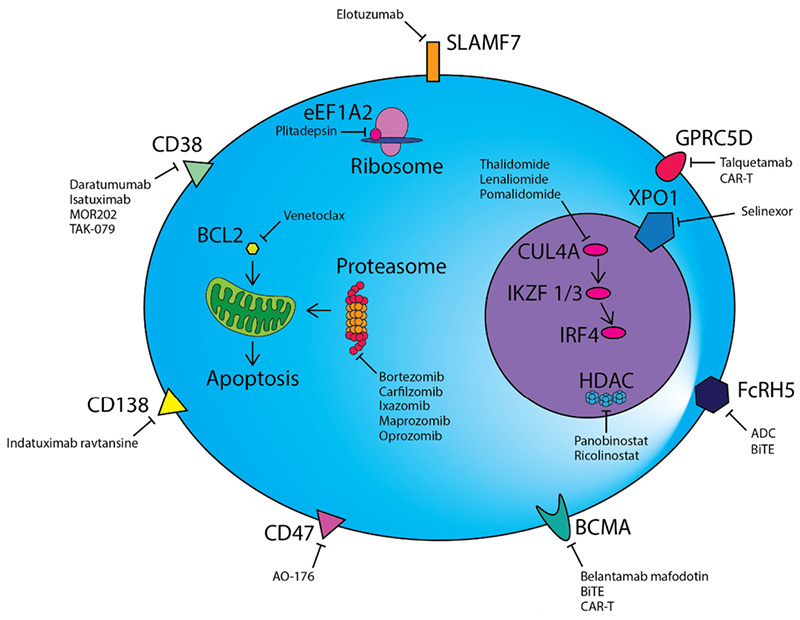
Treatment strategies for MM
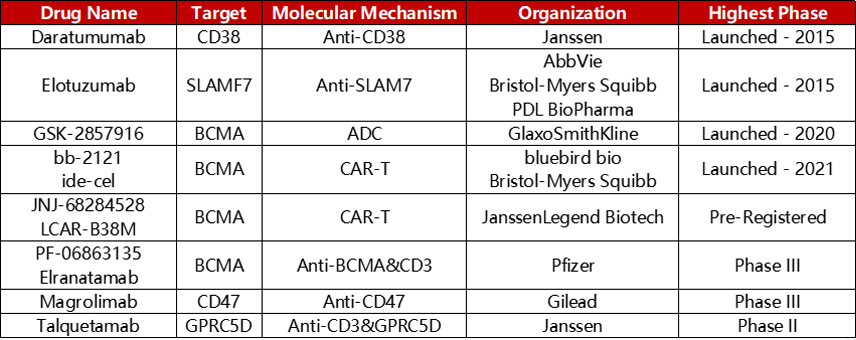
These targets have been clinically developed into a series of targeted drug forms, including monoclonal antibodies, bispecific antibodies, CAR-T, ADC. Leading development of representative drugs such as Daratumumab (CD38, monoclonal antibody), Elotuzumab (SLAMF7, monoclonal antibody), Elranatamab (CD3×BCMA, bispecific antibody), 8PX1X7UG4D (BCMA, CAR-T), GSK-2857916 (BCMA, ADC), and so on.
Representative targeted drugs for the treatment of MM
CD38 is involved in regulating cell migration, receptor-mediated adhesion, and interaction with CD31 or hyaluronic acid. In addition, CD38 also has enzymatic activity, regulates the production of nucleotide metabolites, and participates in the regulation of various cellular functions. CD38 is expressed at a low level on normal myeloid cells, lymphocytes, and non-hematopoietic cells, while it is highly expressed in plasma cells and MM cells. Due to its high specificity, CD38 expression has become a potential target for hematological tumors, especially MM. CD38 monoclonal antibody can bind to CD38 on the surface of target cells with high affinity and induce the death of CD38-positive tumor cells through ADCC, CDC, ADCP, and apoptosis pathways.
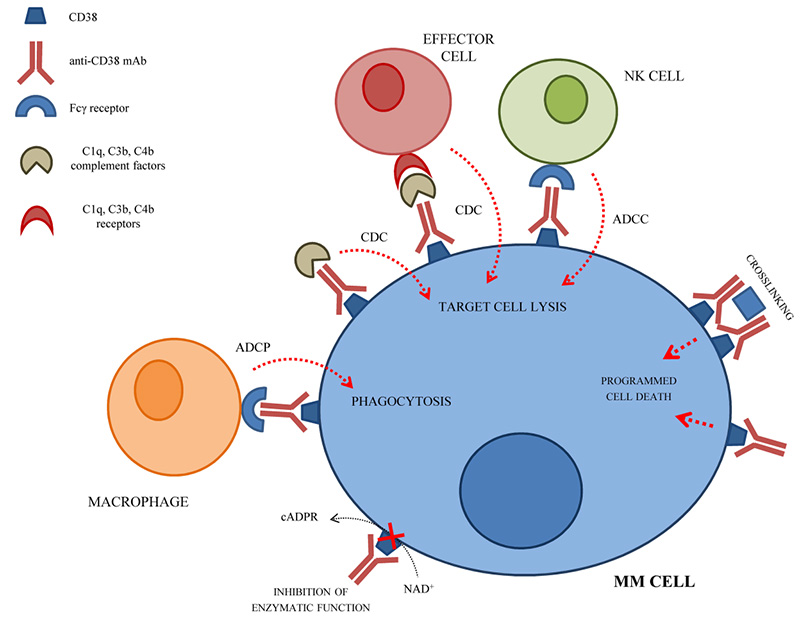
The mechanism of CD38 monoclonal antibody in the treatment of MM
Daratumumab developed by Johnson & Johnson is currently the only CD38 monoclonal antibody product that has been marketed in China. It can be used as a single therapeutic drug or in combination with other anti-tumor agents. Furthermore, there are many other CD38 monoclonal antibodies currently in the research and development stage around the world. In addition, there is evidence that daratumumab causes depletion of CD38+ myeloid-derived suppressor cells and CD38+ regulatory T cells while inducing a clonal expansion of cytotoxic and helper T cells. This enhanced T cell response can potentially be used to increase the binding of T cells to bispecific proteins. Research has begun to add Daratumumab to non-CD38 targeting bispecific proteins (anti-BCMA and anti-GPRC5D) to verify this Hypothesis. The bispecific antibody GBR1342 targeting CD38×CD3 is in phase II clinical trials.
B-cell maturation antigen (BCMA, CD269) expressed at high levels on both malignant plasma cells and normal plasma cells. It is a type III transmembrane glycoprotein in the tumor necrosis factor receptor superfamily. Its function is to regulate B-cell proliferation, maturation, survival, and differentiation to plasma cells through binding
of its ligands, a proliferation-inducing ligand (APRIL), and B-cell–activating factor (BAFF). BCMA is expressed only on mature B lymphocytes, plasmablasts and plasma cells, but not detected in naive B cells and hematopoietic stem cells. Compared with normal plasma cells, the expression of BCMA on malignant plasma cells is increased, and it is upregulated during disease progression from monoclonal gammopathy of undetermined significance to smoldering MM to active MM. The high expression of soluble BCMA is due to the shedding induced by γ-secretase, which is related to the poor prognosis of MM.
The orphan G protein–coupled receptor class C group 5 member D (GPRC5D) is a relatively novel target for MM immunotherapy. Under normal circumstances, its expression is limited to hair follicles, and it is specifically expressed in MM cells. Therefore, GPRC5D is a potential excellent target for the treatment of MM.
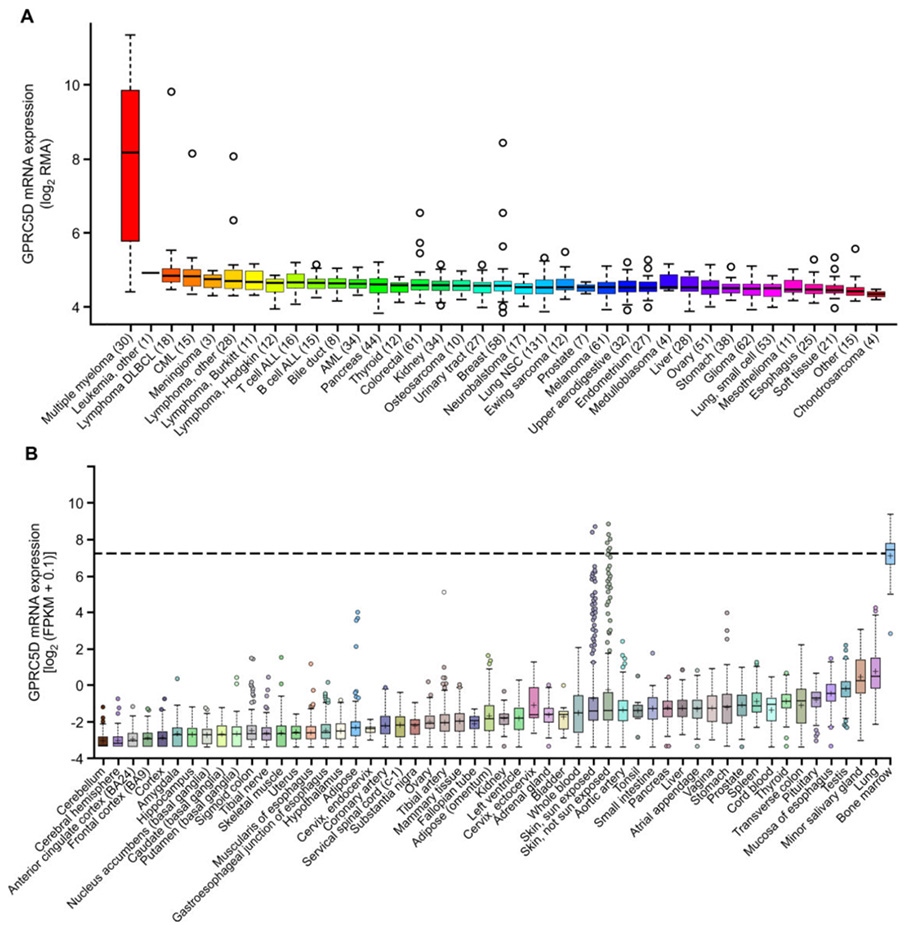
The expression of GPRC5D mRNA in MM and skin
As a newly discovered target, GPRC5D currently has few related research and enterprise layouts. The more advanced is the IgG4 bispecific antibody Talquetamab (JNJ-64407564) developed by Johnson & Johnson that targets GPRC5D×CD3, which activates CD3+T cells and induces T cells to kill GPRC5D+ MM cells.
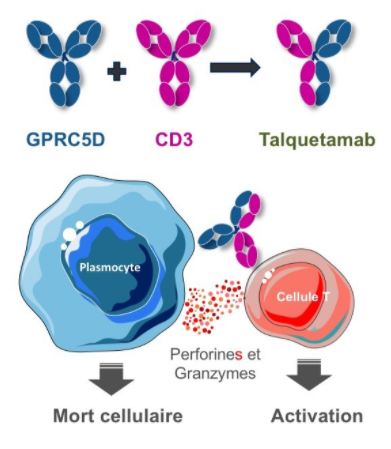
The mechanism action of Talquetamab
Early clinical trials of Talquetamab determined that the recommended dose for phase 2 was 405 µg/kg once a week. When Talquetamab was injected subcutaneously at this dose, the total remission rate was 70%, and the study found that the remission induced by Talquetamab was longer.
Furthermore, preclinical data shows that GPRC5D has better specificity than BCMA. Even in a model where BCMA escape occurs, it can also clear tumors and prolong survival. There is no doubt that GPRC5D has become the next hot target for the treatment of MM.
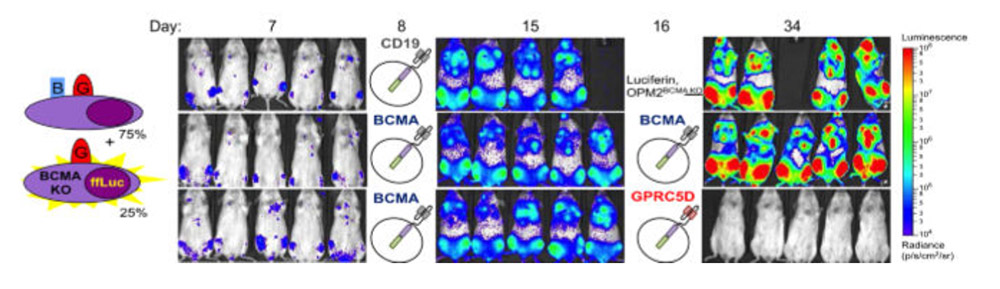
Efficacy of GPRC5D in BCMA recurrence model
GPRC5D is a 7-pass transmembrane protein, and its complex structure poses a huge challenge to the preparation of soluble antigens.
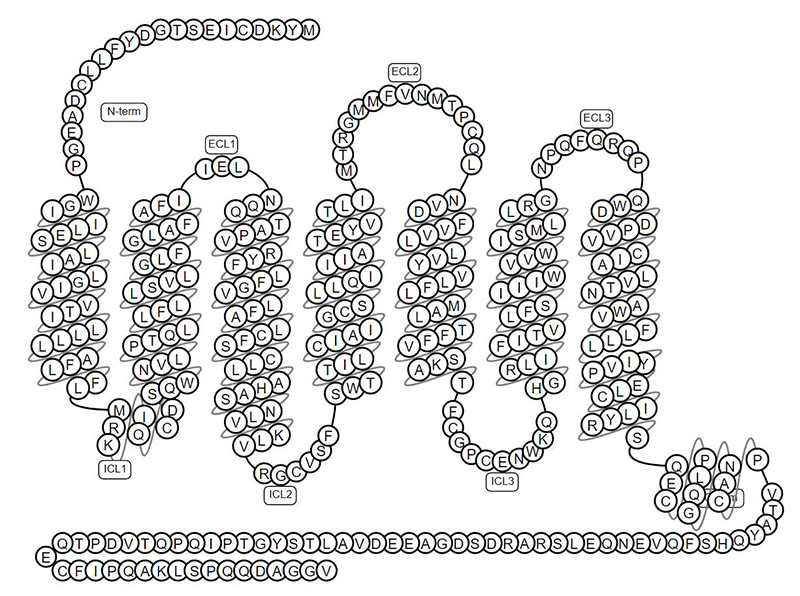
The topology structure of GPRC5D
ACROBiosystems has set up platform-based solutions for developing full-length multi-pass transmembrane proteins. We can provide three versions of GPRC5D including GPRC5D-VLP, GPRC5D-DDM/CHS, and GPRC5D-Nanodisc, all of which have native conformation and complete epitopes. The high biological activity of GPRC5D is verified by binding with anti-GPRC5D antibody via ELISA to meet more applications. Therefore, we are assisting the research and development of GPRC5D targeted drugs and therapies.
Learn more about target proteins for MM immunotherapy
| BCMA | CD38 | CD138 |
| CD47 | GPRC5D | SLAMF7 |
Targeting CD38/CD3 brings breakthroughs in the immunotherapy of multiple myeloma
1. LekhaMikkilineni, James N. Kochenderfer. CAR T cell therapies for patients with multiple myeloma. Nat Rev Clioncol. 2021, 18(2):71-84. https://doi.org/10.1038/s41571-020-0427-6
2. Leow CC, Low MSY. Targeted Therapies for Multiple Myeloma. J Pers Med. 2021 Apr 23;11(5):334. doi: 10.3390/jpm11050334. PMID: 33922567; PMCID: PMC8145732.
3. Smith EL, Harrington K, et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci Transl Med. 2019, 11(485). DOI10.1126/scitranslmed.aau7746
4. Guido Lancman1, Dahniel L. Sastow,et al. Bispecifc Antibodies in Multiple Myeloma: Present and Future.Blood Cancer Discov 2021, 2:423–33. doi: 10.1158/2643-3230.BCD-21-0028
5. https://www.cancernetwork.com/view/talquetamab-shows-durable-and-continuous-response-treating-r-r-multiple-myeloma
This web search service is supported by Google Inc.